Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome
Abstract
:1. Introduction
2. MSC-Derived Secretome as New, Cell-Free Therapeutic Agent in Regenerative Medicine
3. Molecular Mechanisms Responsible for Beneficial Effects of MSC-Derived Secretome
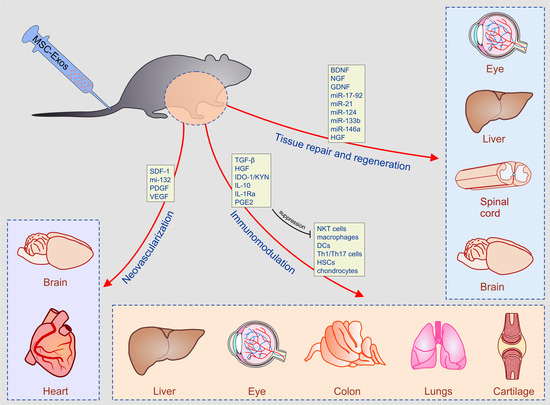
3.1. Immunomodulatory Properties of MSC-Derived Secretome
3.2. The Role of MSC-Sourced Secretome in Tissue Repair and Regeneration
4. Experimental Evidence for Therapeutic Potential of MSC-Derived Secretome in the Treatment of Inflammatory and Degenerative Diseases
4.1. Beneficial Effects of MSC-Derived Secretome in the Treatment of Acute Liver Failure and Liver Fibrosis
4.2. MSC-Sourced Secretome in the Therapy of Lung Diseases
4.3. Therapeutic Potential of MSC-Derived Secretome in Cartilage Regeneration
4.4. Attenuation of Inflammatory Bowel Diseases by MSC-Derived Secretome
4.5. MSC-Sourced Secretome as an Emerging Tool for Myocardial Regeneration
4.6. Beneficial Effects of MSC-Derived Secretome in the Therapy of Eye Disease
4.7. MSC-Derived Secretomes in the Therapy of Ischemic Brain Damage and Spinal Cord Injury
5. Clinical Studies Addressing Therapeutic Potential of MSC-Derived Secretome
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1-MT | 1-methyl-dl-tryptophan |
| ALF | acute liver failure |
| ALI | acute lung injury |
| AT-MSCs | adipose tissue-derived MSCs |
| bFGF | basic fibroblast growth factor |
| BSCB | blood-spinal cord barrier |
| HSP | heat-shock proteins |
| BDNF | brain-derived neurotrophic factor |
| CSCs | cardiac stem cells |
| Cav-1 | caveolin-1 |
| COPD | chronic obstructive pulmonary diseases |
| CD | Crohn’s disease |
| CMV | cytomegalovirus |
| CTLs | cytotoxic T lymphocytes |
| DSS | dextran sodium sulphate |
| DED | dry eye disease |
| ECs | endothelial cells |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| Exos | exosomes |
| EAU | experimental autoimmune uveitis |
| EVs | extracellular vesicles |
| Fap-1 | Fas-associated phosphatase-1 |
| FGF-6 | fibroblast growth factor 6 |
| GCN2 | general control nonderepressible 2 |
| GDNF | glial cell line-derived neurotrophic factor |
| GSH | glutathione |
| GAGs | glycosaminoglycans |
| GRO | growth related oncogene |
| HGF | hepatic growth factor |
| HSCs | hepatic stellate cells |
| HSV | herpes simplex virus |
| hAT-MSC-CM | human adipose tissue MSC-derived conditioned medium |
| hUTC-MSC-CM | human uterine cervical MSC-derived conditioned medium |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| IPF | idiopathic pulmonary fibrosis |
| IL-1Ra | IL-1 receptor antagonist |
| IDO | indolamine 2-3-dioxygenase |
| iNOS | inducible nitric oxide synthase |
| IBDs | inflammatory bowel diseases |
| KGF | keratinocyte growth factor |
| KYN | kynurenine |
| MMIF | macrophage migration inhibitory factor |
| MHC | major histocompatibility complex |
| MDA | malondialdehyde |
| mTOR | mammalian target of rapamycin |
| MMP | matrix metalloproteinase |
| MSCs | mesenchymal stem cells |
| MCP-1 | monocyte chemotactic protein-1 |
| MSC-CM | MSC-derived conditioned medium |
| MTF | MSC-derived trophic factors |
| MPO | Myeloperoxidase |
| NSF | N-ethylmaleimide-sensitive factor |
| NK | natural killer |
| NKT | natural killer T |
| NGF | nerve growth factor |
| nSMase2 | neutral sphingomyelinase 2 |
| NO | nitric oxide |
| NF-κB | nuclear factor κB |
| OA | osteoarthritis |
| OVA | ovalbumin |
| PB-MNCs | peripheral blood mononuclear cells |
| PTEN | phosphatase and tensin homolog |
| PGF | placental growth factor |
| PDGF | platelet-derived growth factor |
| PGE2 | prostaglandin E2 |
| RhoA | Ras homolog gene family member A |
| RGCs | retinal ganglion cells |
| S1P | sphingosine 1-phosphate |
| SCI | spinal cord injury |
| SOD | superoxide dismutase |
| TSP1 | thrombospondin 1 |
| TIMP-1 | tissue inhibitors of metalloproteinase |
| TGF-β | transforming growth factor-β |
| Tregs | T regulatory cells |
| TNFSF14 | tumor necrosis factor superfamily member 14 |
| UC | ulcerative colitis |
| VEGF | vascular endothelial growth factor |
References
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E., 2nd; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W., Jr.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest. 2017, 151, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.O.; Allez, M.; Clark, M.; Labopin, M.; Ricart, E.; Rogler, G.; Rovira, M.; Satsangi, J.; Farge, D.; Hawkey, C.J.; ASTIC Trial Group; European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party; European Crohn’s and Colitis Organisation. Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: An analysis of pooled data from the ASTIC trial. Lancet Gastroenterol. Hepatol. 2017, 2, 399–406. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. (Lond.) 2005, 2. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal stem cells: A friend or foe in immune-mediated diseases. Stem Cell Rev. 2015, 11, 280–287. [Google Scholar] [CrossRef]
- Meier, R.P.; Müller, Y.D.; Morel, P.; Gonelle-Gispert, C.; Bühler, L.H. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013, 11, 1348–1364. [Google Scholar] [CrossRef] [PubMed]
- Soukup, T.; Mokrý, J.; Karbanová, J.; Pytlík, R.; Suchomel, P.; Kucerová, L. Mesenchymal stem cells isolated from the human bone marrow: Cultivation, phenotypic analysis and changes in proliferation kinetics. Acta Medica (Hradec Kralove) 2006, 49, 27–33. [Google Scholar] [CrossRef]
- Gou, S.; Wang, C.; Liu, T.; Wu, H.; Xiong, J.; Zhou, F.; Zhao, G. Spontaneous differentiation of murine bone marrow-derived mesenchymal stem cells into adipocytes without malignant transformation after long-term culture. Cells Tissues Organs 2010, 191, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Shi, S.; Zhou, S.; Wang, X.; Nie, S. Aspirin inhibits growth and enhances cardiomyocyte differentiation of bone marrow mesenchymal stem cells. Eur. J. Pharmacol. 2018, 827, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Mikael, P.E.; Willard, C.; Koyee, A.; Barlao, C.G.; Liu, X.; Han, X.; Ouyang, Y.; Xia, K.; Linhardt, R.J.; Dordick, J.S. Remodeling of Glycosaminoglycans During Differentiation of Adult Human Bone Mesenchymal Stromal Cells Toward Hepatocytes. Stem Cells Dev. 2019, 28, 278–289. [Google Scholar] [CrossRef]
- Urrutia, D.N.; Caviedes, P.; Mardones, R.; Minguell, J.J.; Vega-Letter, A.M.; Jofre, C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: An approach for their use in neural regeneration therapies. PLoS ONE 2019, 14, e0213032. [Google Scholar] [CrossRef]
- Jiao, X.; Lv, Q.; Cao, S.N. MicroRNA-26b-5p promotes development of neonatal respiratory distress syndrome by inhibiting differentiation of mesenchymal stem cells to type II of alveolar epithelial cells via regulating Wnt5a. Eu.r Rev. Med. Pharmacol. Sci. 2019, 23, 1681–1687. [Google Scholar] [CrossRef]
- Lou., G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef] [PubMed]
- Gazdic, M.; Arsenijevic, A.; Markovic, B.S.; Volarevic, A.; Dimova, I.; Djonov, V.; Arsenijevic, N.; Stojkovic, M.; Volarevic, V. Mesenchymal Stem Cell-Dependent Modulation of Liver Diseases. Int. J. Biol. Sci. 2017, 13, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar] [CrossRef]
- Xagorari, A.; Siotou, E.; Yiangou, M.; Tsolaki, E.; Bougiouklis, D.; Sakkas, L.; Fassas, A.; Anagnostopoulos, A. Protective effect of mesenchymal stem cell-conditioned medium on hepatic cell apoptosis after acute liver injury. Int. J. Clin. Exp. Pathol. 2013, 6, 831–840. [Google Scholar]
- Parekkadan, B.; van Poll, D.; Megeed, Z.; Kobayashi, N.; Tilles, A.W.; Berthiaume, F.; Yarmush, M.L. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 363, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.; Gazdic, M.; Simovic Markovic, B.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Jovicic, N.; Jeftic, I.; Djonov, V.; Arsenijevic, N.; et al. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells—An experimental study. Transpl. Int. 2018, 31, 102–115. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Takeoka, M.; Ward, W.F.; Pollack, H.; Kamp, D.W.; Panos, R.J. KGF facilitates repair of radiation-induced DNA damage in alveolar epithelial cells. Am. J. Physiol. 1997, 272, L1174–L1180. [Google Scholar] [CrossRef]
- Li, J.W.; Wu, X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2368–2378. [Google Scholar]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.H.; Kim, H.J.; Park, M.K.; Huh, J.W.; Ro, J.Y.; Oh, Y.M.; Lee, S.D.; Lee, Y.S. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2012, 302, L891–L908. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.Y.; Cho, R.; Shin, D.M.; Lee, S.W.; Oh, Y.M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017, 49, e284. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin. Cell. Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- Mao, F.; Wu, Y.; Tang, X.; Kang, J.; Zhang, B.; Yan, Y.; Qia, H.; Zhang, X.; Xu, W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.X.; Fan, H.; Tang, Q.; Shou, Z.X.; Zuo, D.M.; Zou, Z.; Xu, M.; Chen, Q.Y.; Peng, Y.; et al. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS ONE 2015, 10, e0140551. [Google Scholar] [CrossRef]
- Hetzenecker, A.M.; Seidl, M.C.; Kosovac, K.; Herfarth, H.; Kellermeier, S.; Obermeier, F.; Falk, W.; Schoelmerich, J.; Hausmann, M.; Rogler, G. Downregulation of the ubiquitin-proteasome system in normal colonic macrophages and reinduction in inflammatory bowel disease. Digestion 2012, 86, 34–47. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Dovendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef]
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631. [Google Scholar] [CrossRef]
- Vishnubhatla, I.; Corteling, R.; Stevanato, L.; Hicks, C.; Sinden, J. The Development of Stem Cell-Derived Exosomes as a Cell-Free Regenerative Medicine. J. Circ. Biomark. 2014, 3. [Google Scholar] [CrossRef]
- Bright, J.J.; Kerr, L.D.; Sriram, S. TGF-beta inhibits IL-2-induced tyrosine phosphorylation and activation of Jak-1 and Stat 5 in T lymphocytes. J. Immunol. 1997, 159, 175–183. [Google Scholar] [PubMed]
- Harrell, C.R.; Simovic Markovic, B.; Fellabaum, C.; Arsenijevic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. Adv. Exp. Med. Biol. 2018, 1089, 47–57. [Google Scholar] [CrossRef]
- Fan, Y.; Herr, F.; Vernochet, A.; Mennesson, B.; Oberlin, E.; Durrbach, A. Human Fetal Liver Mesenchymal Stem Cell-Derived Exosomes Impair Natural Killer Cell Function. Stem Cells Dev. 2019, 28, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yu, L.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J. Cell. Physiol. 2018, 233, 6832–6840. [Google Scholar] [CrossRef]
- Acovic, A.; Gazdic, M.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Arsenijevic, N.; Volarevic, V. Role of indoleamine 2,3-dioxygenase in pathology of the gastrointestinal tract. Therap. Adv. Gastroenterol. 2018, 11. [Google Scholar] [CrossRef]
- Showalter, M.R.; Wancewicz, B.; Fiehn, O.; Archard, J.A.; Clayton, S.; Wagner, J.; Deng, P.; Halmai, J.; Fink, K.D.; Bauer, G.; et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem. Biophys. Res. Commun. 2019, 512, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, F.; He, X.; Yang, X.; Shan, F.; Feng, J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 2019, 67, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xiao, Y.; Xiao, Y.; Guo, Q.; Li, C.; Huang, Y.; Deng, Q.; Wen, J.; Zhou, F.; Luo, X.H. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano 2019, 13, 2450–2462. [Google Scholar] [CrossRef]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell. Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Mahmoudi, M.; Kalantari, M.; Taghdisi, S.M.; Ramezani, M. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J. Control. Release 2019, 299, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019, 5126156. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Guo, R.; Zhang, Y.; Chen, D.; Li, X.; Tian, W.; Xie, X.; Jiang, Z. Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life 2019. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Al-Qahtani, A.; Arsenijevic, N.; Pajovic, S.; Lukic, M.L. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 2010, 43, 255–263. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef]
- Harting, M.T.; Srivastava, A.K.; Zhaorigetu, S.; Bair, H.; Prabhakara, K.S.; Toledano Furman, N.E.; Vykoukal, J.V.; Ruppert, K.A.; Cox, C.S., Jr.; Olson, S.D. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells 2018, 36, 79–90. [Google Scholar] [CrossRef]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors 2017, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Alcoholado, C.; Martín-Astorga, M.C.; Fernández, V.; Cifuentes, M.; Becerra, J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet. Immunol. Immunopathol. 2019, 208, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Zagoura, D.S.; Roubelakis, M.G.; Bitsika, V.; Trohatou, O.; Pappa, K.I.; Kapelouzou, A.; Antsaklis, A.; Anagnou, N.P. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 2012, 61, 894–906. [Google Scholar] [CrossRef]
- Xiong, L.L.; Li, Y.; Shang, F.F.; Chen, S.W.; Chen, H.; Ju, S.M.; Zou, Y.; Tian, H.L.; Wang, T.H.; Luo, C.Z.; et al. Chondroitinase administration and pcDNA3.1-BDNF-BMSC transplantation promote motor functional recovery associated with NGF expression in spinal cord-transected rat. Spinal Cord 2016, 54, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Angioni, R.; Calì, B.; Soldani, C.; Ploia, C.; Moalli, F.; Gargesha, M.; D’Amico, G.; Elliman, S.; Tedeschi, G.; et al. Mouse mesenchymal stem cells inhibit high endothelial cell activation and lymphocyte homing to lymph nodes by releasing TIMP-1. Leukemia 2016, 30, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Zhang, Z.; Jiang, Z. Hypoxia promotes bone marrow-derived mesenchymal stem cell proliferation through apelin/APJ/autophagy pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2015, 47, 362–367. [Google Scholar] [CrossRef]
- Jakovljevic, J.; Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Jovicic, N.; Volarevic, V. Modulation of autophagy as new approach in mesenchymal stem cell-based therapy. Biomed. Pharmacother. 2018, 104, 404–410. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell. Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef]
- Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef]
- Eiró, N.; Sendon-Lago, J.; Seoane, S.; Bermúdez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Cheng, X.; Wang, H.; Huang, W.; la Ga Hu, Z.; Wang, D.; Zhang, K.; Zhang, H.; Xue, Z.; Da, Y.; et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J. Transl. Med. 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Mansurov, N.; Chen, W.C.W.; Awada, H.; Huard, J.; Wang, Y.; Saparov, A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue. Eng. Regen. Med. 2018, 12, e1164–e1172. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.; Gazdic, M.; Simovic Markovic, B.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Djonov, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 2017, 23, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, C.; Oo, Y.H.; Murphy, N.; Adams, D.H.; Lalor, P.F. The regulation of T-cell recruitment to the human liver during acute liver failure. Liver Int. 2013, 33, 852–863. [Google Scholar] [CrossRef]
- Gazdic, M.; Simovic Markovic, B.; Vucicevic, L.; Nikolic, T.; Djonov, V.; Arsenijevic, N.; Trajkovic, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase- and indoleamine 2,3-dioxygenase-dependentmanner. J. Tissue Eng. Regen. Med. 2018, 12, e1173–e1185. [Google Scholar] [CrossRef] [PubMed]
- Fabre, T.; Molina, M.F.; Soucy, G.; Goulet, J.P.; Willems, B.; Villeneuve, J.P.; Bilodeau, M.; Shoukry, N.H. Type 3 cytokines IL-17A and IL-22 drive TGF-β-dependent liver fibrosis. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef]
- Fabre, T.; Kared, H.; Friedman, S.L.; Shoukry, N.H. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 2014, 193, 3925–3933. [Google Scholar] [CrossRef]
- Owen, A.; Newsome, P.N. Mesenchymal stromal cell therapy in liver disease: Opportunities and lessons to be learnt? Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G791–G800. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; Xu, W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Wang, S.; Kim, J.; Kim, G.J.; Jung, Y. MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- De Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.M.; Zhuansun, Y.X.; Chen, R.; Lin, L.; Lin, Y.; Li, J.G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018, 363, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, C.; Cao, H.; Chen, L.; Hou, J.; Xiang, Z.; Hu, K.; Han, X. The hedgehog and Wnt/β-catenin system machinery mediate myofibroblast differentiation of LR-MSCs in pulmonary fibrogenesis. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, C.; Ji, H.L. Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses. Stem Cells Transl. Med. 2019, 8, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Shentu, T.P.; Huang, T.S.; Cernelc-Kohan, M.; Chan, J.; Wong, S.S.; Espinoza, C.R.; Tan, C.; Gramaglia, I.; van der Heyde, H.; Chien, S.; et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, H.S.; Hong, I.S. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, Y.W.; Tan, K.P.; Shen, Y.S.; Wang, Y.H.; Chang, C.H. Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS ONE 2018, 13, e0205563. [Google Scholar] [CrossRef] [PubMed]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, L.; Zou, R.; Wen, C.; Wang, Z.; Lin, F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 2018, 17, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zou, R.; Wang, Z.; Wen, C.; Zhang, F.; Lin, F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J. 2018, 475, 3629–3638. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Colgan, S.P.; Eltzschig, H.K.; Eckle, T.; Thompson, L.F. Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal. 2006, 2, 351–360. [Google Scholar] [CrossRef]
- Sun, H.; Hu, S.; Zhang, Z.; Lun, J.; Liao, W.; Zhang, Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell. Biochem. 2019, 120, 171–181. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhao, G.Z.; Xu, X.Y.; Fang, J.; Chen, J.M.; Li, J.W.; Gao, X.J.; Hao, L.J.; Chen, Y.Z. Integrin-β1 regulates chondrocyte proliferation and apoptosis through the upregulation of GIT1 expression. Int. J. Mol. Med. 2015, 35, 1074–1080. [Google Scholar] [CrossRef]
- Herreros, M.D.; Garcia-Arranz, M.; Guadalajara, H.; De-La-Quintana, P.; Garcia-Olmo, D.; FATT Collaborative Group. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: A phase III randomized clinical trial (FATT 1: Fistula Advanced Therapy Trial 1) and long-term evaluation. Dis. Colon. Rectum 2012, 55, 762–772. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, K.J.; Cho, Y.B.; Yoon, S.N.; Song, K.H.; Kim, D.S.; Jung, S.H.; Kim, M.; Yoo, H.W.; Kim, I.; et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 2013, 31, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.B.; Lee, W.Y.; Park, K.J.; Kim, M.; Yoo, H.W.; Yu, C.S. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: A phase I clinical study. Cell Transplant. 2013, 22, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, K.; Tokhanbigli, S.; Asadzadeh, H.; Nmaki, S.; Reza Zali, M.; Hashemi, S.M. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J. Cell Physiol. 2019, 234, 9910–9926. [Google Scholar] [CrossRef]
- Capitini, C.M.; Chisti, A.A.; Mackall, C.L. Modulating T-cell homeostasis with IL-7: Preclinical and clinical studies. J. Intern. Med. 2009, 266, 141–153. [Google Scholar] [CrossRef]
- Ji, T.; Xu, C.; Sun, L.; Yu, M.; Peng, K.; Qiu, Y.; Xiao, W.; Yang, H. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig. Dis. Sci. 2015, 60, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiu, W.; Xu, X.; Kang, J.; Wang, J.; Wen, Y.; Tang, X.; Yan, Y.; Qian, H.; Zhang, X.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am. J. Transl. Res. 2018, 10, 2026–2036. [Google Scholar]
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem cell-derived exosomes-an emerging tool for myocardial regeneration. World J. Stem Cells 2018, 10, 106–115. [Google Scholar] [CrossRef]
- Ju, C.; Shen, Y.; Ma, G.; Liu, Y.; Cai, J.; Kim, I.M.; Weintraub, N.L.; Liu, N.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 420–428. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Yan, W.; Li, Y.; Shen, Z.; Asahara, T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Hu, C.F.; Li, R.; Zhou, Z.; Shen, C.X. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cell. Physiol. Biochem. 2017, 43, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; He, Z.; Liang, Z.; Chen, Z.; Wang, H.; Zhang, J. Exosomes From Adipose-derived Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Wnt/β-Catenin Signaling Pathway. J. Cardiovasc. Pharmacol. 2017, 70, 225–231. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef]
- Suzuki, E.; Fujita, D.; Takahashi, M.; Oba, S.; Nishimatsu, H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World. J. Stem Cells 2016, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Dimova, I.; Karthik, S.; Makanya, A.; Hlushchuk, R.; Semela, D.; Volarevic, V.; Djonov, V. SDF-1/CXCR4 signalling is involved in blood vessel growth and remodelling by intussusception. J. Cell Mol. Med. 2019. [Google Scholar] [CrossRef]
- Gong, X.H.; Liu, H.; Wang, S.J.; Liang, S.W.; Wang, G.G. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J. Cell Physiol. 2019. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Chen, Y.; Meng, Q.; Sun, J.; Shao, L.; Yu, Y.; Huang, H.; Hu, Y.; Yang, Z.; et al. MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Tian, X.; Li, Q.; Yang, Y. New strategies for improving stem cell therapy in ischemic heart disease. Heart Fail. Rev. 2016, 21, 737–752. [Google Scholar] [CrossRef]
- Parikh, M.; Raj, P.; Yu, L.; Stebbing, J.A.; Prashar, S.; Petkau, J.C.; Tappia, P.S.; Pierce, G.N.; Siow, Y.L.; Brown, D.; et al. Ginseng Berry Extract Rich in Phenolic Compounds Attenuates Oxidative Stress but not Cardiac Remodeling post Myocardial Infarction. Int. J. Mol. Sci. 2019, 20, 983. [Google Scholar] [CrossRef]
- Sun, X.; Shan, A.; Wei, Z.; Xu, B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2018, 503, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Invest. Ophthalmol. Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Invest. Ophthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.K.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Reports. 2017, 8, 1214–1225; [Google Scholar] [CrossRef]
- Gayton, J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D., 3rd; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal. Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef]
- Lu, P.; Li, L.; Liu, G.; Zhang, X.; Mukaida, N. Enhanced experimental corneal neovascularization along with aberrant angiogenic factor expression in the absence of IL-1 receptor antagonist. Invest. Ophthalmol. Vis. Sci. 2009, 50, 4761–4768. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Simovic Markovic, B.; Arsenijevic, A.; Volarevic, V. Therapeutic potential of “Exosomes derived Multiple Allogeneic Proteins Paracrine Signaling: Exosomes d-MAPPS” is based on the effects of exosomes, immunosuppressive and trophic factors. Ser. J. Exp. Clin. Res. 2018. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Caterson, B.; Kao, W.W. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells 2013, 31, 2116–2126. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, P.; Yao, X.; Li, H.; Shen, H.; Li, X.; Wu, J.; Lu, X. Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Huang, J.H.; Yin, X.M.; Xu, Y.; Xu, C.C.; Lin, X.; Ye, F.B.; Cao, Y.; Lin, F.Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma 2017, 34, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Wang, L.; Pei, S.; Han, L.; Guo, B.; Li, Y.; Duan, R.; Yao, Y.; Xue, B.; Chen, X.; Jia, Y. Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFκB P65 Subunit in Spinal Cord Injury. Cell Physiol. Biochem. 2018, 50, 1535–1559. [Google Scholar] [CrossRef]
- Lankford, K.L.; Arroyo, E.J.; Nazimek, K.; Bryniarski, K.; Askenase, P.W.; Kocsis, J.D. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE 2018, 13, e0190358. [Google Scholar] [CrossRef]
- Shiue, S.J.; Rau, R.H.; Shiue, H.S.; Hung, Y.W.; Li, Z.X.; Yang, K.D.; Cheng, J.K. Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats. Pain 2019, 160, 210–223. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Zhang, R.; Wen, L.; Wu, K.; Li, Y.; Yao, Y.; Duan, R.; Jia, Y. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Recovery Following Spinal Cord Injury via Improvement of the Integrity of the Blood-Spinal Cord Barrier. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016, 12. [Google Scholar] [CrossRef]
- Fukuoka, H.; Suga, H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: Follow-up with trichograms. Eplasty 2015, 15, e10. [Google Scholar]
- Shin, H.; Ryu, H.H.; Kwon, O.; Park, B.S.; Jo, S.J. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. Int. J. Dermatol. 2015, 54, 730–735. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Ringden, O.; Le Blanc, K. Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract. Res. Clin. Haematol. 2011, 24, 65–72. [Google Scholar] [CrossRef]
- Berglund, A.K.; Schnabel, L.V. Allogeneic major histocompatibility complex-mismatched equine bone marrow-derived mesenchymal stem cells are targeted for death by cytotoxic anti-major histocompatibility complex antibodies. Equine Vet. J. 2017, 49, 539–544. [Google Scholar] [CrossRef]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 2015, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Ryan, A.E.; Alagesan, S.; Lohan, P.; Treacy, O.; Ritter, T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: What have we learned so far? Immunol. Cell Biol. 2013, 91, 40–51. [Google Scholar] [CrossRef]
- Liu, Q.; Rojas-Canales, D.M.; Divito, S.J.; Shufesky, W.J.; Stolz, D.B.; Erdos, G.; Sullivan, M.L.; Gibson, G.A.; Watkins, S.C.; Larregina, A.T.; et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Invest. 2016, 126, 2805–2820. [Google Scholar] [CrossRef]







| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| BM-MSCs | Exos | leukocytes/ALF and liver fibrosis | inhibited activation of inflammasome | IDO-1/KYN; TGF-β; IL-10 | [13,74] |
| AT-MSCs | Exos | HSCs/liver fibrosis | reduced collagen production | miR-122:Hedgehog/Smoothened | [13] |
| BM-MSCs | CM | macrophages/liver fibrosis | conversion from M1 to M2 phenotype | TGF-β/Smad | [14] |
| BM-MSC | CM | Th1 and Th17 cells/ALF | reduced influx in the injured liver | IL-10; CXCR3 and CCR5 | [15,75] |
| BM-MSC | CM/Exos | hepatocytes/ALF | inhibition of apoptosis and enhanced proliferation | IDO-1/KYN; HGF; fibrinogen-like protein 1; IL-6/gp130; Bcl-xL; Cyclin D1 | [16,17,74,76] |
| BM-MSC | CM | NKT cells/ALF | reduced production of inflammatory cytokines and attenuated cytotoxicity | IDO-1/KYN | [74,76] |
| BM-MSCs | CM | T cells/liver fibrosis | expansion of Tregs; suppression of Th17 cells | IDO-1/KYN | [18] |
| BM-MSCs | CM | HSCs/liver fibrosis | inhibited activation and enhanced apoptosis | IDO-1/KYN; IL-10; NGF/p75 | [77,78] |
| UCD-MSCs | Exos | HSCs/liver fibrosis | reduced collagen production | TGF-β/Smad2 | [80] |
| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| BM-MSCs | EVs | alveolar type II epithelial cells/ALI | attenuation of oxidant-mediated injury | KGF | [19,20,21,22] |
| BM-MSCs | Exos | alveolar macrophages/ALI | generation of immunosuppressive phenotype | IL-10; TGF-β | [83] |
| AT-MSCs/BM-MSCs | CM/Exos | neutrophils; eosinophils; Th2 and Th17 cells/asthma | suppression of cytokine production | IL-10 | [84,85] |
| BM-MSCs | Exos | DCs/asthma | attenuated antigen presenting function | IL-10; TGF-β | [86] |
| BM-MSCs/AT-MSCs | CM | alveolar type II epithelial cells; fibroblasts/COPD | attenuated apoptosis; suppressed collagen deposition | FGF-2 | [23,24,25] |
| BM-MSCs | EVs | fibroblasts/IPF | suppressed myofibroblastic differentiation | miR-630 | [89,91] |
| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| BM-MSCs/AT-MSCs | CM/Exos | chondrocytes/in vitro | reduced production of inflammatory cytokines | IL-10 | [92,93] |
| BM-MSCs | Exos | chondrocytes/OA | restoration of homeostasis in bioenergetics and cell metabolism/restoration of cartilage and subchondral bone | adenylate kinase and nucleoside-diphosphate kinase-dependent pathways | [26,27] |
| BM-MSCs | Exos | chondrocytes/OA | enhanced proliferation/cartilage regeneration | miR-320c and miR-92a–3p | [98,99] |
| BM-MSCs | Exos | chondrocytes/OA | reduced apoptosis and enhanced proliferation/cartilage regeneration | KLF3-AS1 | [100] |
| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| UCD-MSCs | Exos | macrophages/colitis | reduced production of inflammatory cytokines | IL-10/IL-7 | [28] |
| BM-MSCs | EVs | leukocytes/colitis | reduced production of inflammatory cytokines | NF-kB-p65 | [29] |
| BM-MSCs | EVs | epithelial cell/colitis | attenuation of oxidative stress and inhibition of apoptosis | MPO, MDA, SOD, GSH/caspase-3,-8 and -9 | [29] |
| BM-MSCs | Exos | epithelial cell/colitis | down-regulated expression of ubiquitin and ubiquitin-associated molecules (K48, K63 and FK2) | IL-10; IDO-1/KYN | [30] |
| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| BM-MSCs | Exos | cardiomyocytes/myocardial ischemia/reperfusion injury | improved contractility/reduced infarct size | PI3K/Akt | [31,32,33] |
| BM-MSCs | Exos | cardiomyocytes/myocardial ischemia/reperfusion injury | prevention of apoptosis and induction of autophagy/reduced myocardial infarct size | AMPK/mTOR and Akt/mTOR | [111] |
| BM-MSCs | Exos | cardiomyocytes/myocardial ischemia/reperfusion injury | prevention of apoptosis and increased survival/reduced myocardial infarct size | Bcl-2, Bax, caspase-3; Wnt/β-catenin | [112] |
| BM-MSCs | Exos | cardiomyocytes/myocardial ischemia/reperfusion injury | increased survival/reduced infarct size | miR-210 and miR-125b-5p | [113,114] |
| BM-MSCs | Exos | cardiomyocytes/myocardial ischemia/reperfusion injury | higher survival/smaller scar size/better cardiac function | neutral sphingomyelinase /miR-210 | [114] |
| BM-MSCs | Exos | endothelial cells/ischemia/reperfusion injury | generation of new blood vessels in peri-infarcted myocardial zone | SDF-1/miR-132 | [117,118] |
| BM-MSCs | Exos | cardiac stem cells/in vitro/ischemia/reperfusion injury | prevention of apoptosis and increased survival and proliferation | miR-15, miR-21, miR-22, miR-126, miR-146a, miR-210 | [119,120] |
| BM-MSCs | Exos | cardiomyocytes/macrophages/dilated cardiomyopathy | reduced apoptosis of cardiomyocytes/reduced production of inflammatory cytokines in macrophages//improved myocardial function/attenuated cardiac dilation | IL-10/VEGF | [121] |
| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| BM-MSCs | Exos | RGCs/glaucoma | Increased survival and regeneration of RGCs/attenuation of glaucoma | BDNF, NGF, PDGF, miR-17-92, miR-21 and miR146a | [122,123,124] |
| BM-MSCs | Exos | macrophages/laser-induced retinal injury | attenuated activation of inflammatory macrophages/alleviation of retinal inflammation/increased number of photoreceptor cells | MCP-1 | [34] |
| BM-MSCs | Exos | neutrophils, NK cells, macrophages and T cells/EAU | reduced influx of inflammatory cells/attenuation of EAU | MCP-1; CCl21 | [35] |
| BM-MSCs | Exos | DCs, Th1, Th17 cells/EAU | attenuation of antigen-presenting function of DCs; reduced production of Th1 and Th17-related cytokines/attenuation of EAU | IL-10; IDO-1/KYN | [125] |
| BM-MSCs | CM/Exos | macrophages; Th1 and Th17 cells/corneal injury; DED | reduced production of IL-1β/attenuated activation of Th1/Th17 cells/alleviated corneal inflammation | IL-1Ra; GRO; IOD-1/KYN | [126,127,128,129,130] |
| UCD-MSCs | Exos | keratocytes/Sly syndrome | enhanced degradation of GAGs/attenuation of Sly syndrome | β-glucuronidase-induced degradation of GAGs | [132] |
| Source Cell Type | Type of Secretome | Target Cells/Condition | Effects/Major Findings | Pathways Involved | Ref. No. |
|---|---|---|---|---|---|
| BM-MSCs | Exos | neurons/ischemic brain injury | improved neurogenesis and neurite remodeling | miR-124 and miR-133b | [36] |
| BM-MSCs | Exos | neurons/SCI | enhanced regeneration of axons | miR-133b/Erk1/2 and Stat-3 | [134] |
| BM-MSCs | Exos | N1 astrocytes/SCI | suppressed production of inflammatory cytokines | IL-10/NF-kB-p65 | [135,137] |
| BM-MSCs | Exos | macrophages/SCI | conversion from inflammatory M1 to immunosuppressive M2 phenotype | IL-10/NF-kB-p65 | [138] |
| UCD-MSCs | Exos | neurons; glial cells/nerve-injury induced pain | reduced excitation of neurons, activation of glial cells/attenuation of nerve-injury induced pain | BDNF, GDNF/IL-10 | [139] |
| BM-MSCs | EVs | pericytes/SCI | reduced migratory capacities of pericytes/increased integrity of BSCB/improved motor function | NF-kB-p65 | [140] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. https://doi.org/10.3390/cells8050467
Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019; 8(5):467. https://doi.org/10.3390/cells8050467
Chicago/Turabian StyleHarrell, Carl Randall, Crissy Fellabaum, Nemanja Jovicic, Valentin Djonov, Nebojsa Arsenijevic, and Vladislav Volarevic. 2019. "Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome" Cells 8, no. 5: 467. https://doi.org/10.3390/cells8050467
APA StyleHarrell, C. R., Fellabaum, C., Jovicic, N., Djonov, V., Arsenijevic, N., & Volarevic, V. (2019). Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells, 8(5), 467. https://doi.org/10.3390/cells8050467