Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes
Abstract
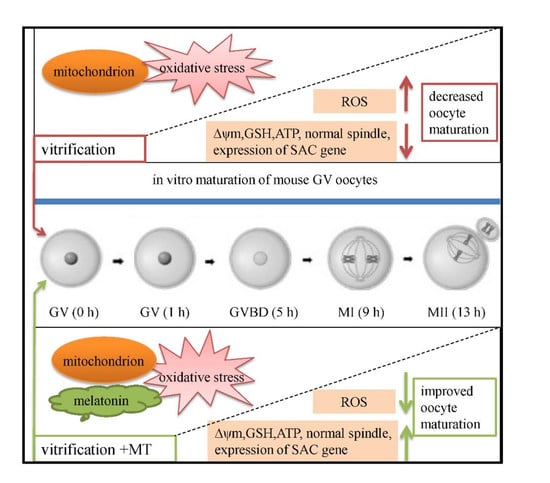
:1. Introduction
2. Materials and Methods
2.1. Oocyte Collection
2.2. Experimental Groups and Screening of Optimal Concentration of Melatonin
2.3. Oocyte Vitrification and Warming
2.4. Oocyte Culture and In Vitro Maturation
2.5. Detection of Mitochondrial Membrane Potential
2.6. Detection of ATP Content in Oocytes
2.7. Spindle Morphology and Classification
2.8. Measurement of Intracellular ROS and GSH Levels
2.9. Quantitative Polymerase Chain Reaction (Q-PCR)
2.10. Statistical Analyses
3. Results
3.1. Effect of Different Concentrations of Melatonin on In Vitro Maturation of Vitrified-Warmed Mouse GV Oocytes
3.2. Melatonin Supplementation Ameliorates ROS Levels in Vitrified-Warmed Mouse GV Oocytes and Their In Vitro-Derived MI and MII Stage Oocytes
3.3. Melatonin Supplementation Ameliorates GSH Levels in Vitrified-Warmed Mouse GV Oocytes and Their In Vitro-Derived MI and MII Stage Oocytes
3.4. Effect of Melatonin Supplementation on Mitochondrial Membrane Potential in Vitrified-Warmed Mouse GV Oocytes and Their In Vitro-Derived MI and MII Stage Oocytes
3.5. Effect of Melatonin Supplementation on ATP Levels in Vitrified-Warmed Mouse GV Oocytes and Their Resultant MI and MII Oocytes
3.6. Effect of Melatonin Supplementation on Spindle Configuration of MII Oocytes Derived from the Vitrified-Warmed Mouse GV Oocytes
3.7. Effect of Melatonin on Expression of SAC-Related Genes in Vitrified-Warmed mouse GV Oocytes and Their In Vitro-Derived Oocytes at GVBD, MI, and MII Stages
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pan, B.; Yang, H.; Wu, Z.; Qazi, I.H.; Liu, G.; Han, H.; Meng, Q.; Zhou, G. Melatonin Improves Parthenogenetic Development of Vitrified—Warmed Mouse Oocytes Potentially by Promoting G1/S Cell Cycle Progression. Int. J. Mol. Sci. 2018, 19, 4029. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology 2007, 67, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H. Cryopreservation of ova and embryos from livestock: Current status and research needs. Theriogenology 1991, 35, 109–124. [Google Scholar] [CrossRef]
- Fabbri, R.; Porcu, E.; Marsella, T.; Rocchetta, G.; Venturoli, S.; Flamigni, C. Human oocyte cryopreservation: New perspectives regarding oocyte survival. Hum. Reprod. 2001, 16, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Mandawala, A.; Harvey, S.; Roy, T.; Fowler, K. Cryopreservation of animal oocytes and embryos: Current progress and future prospects. Theriogenology 2016, 86, 1637–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oktay, K.; Bedoschi, G. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. J. Pediatric Adolesc. Gynecol. 2014, 27, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Kotera, T.; Shibata, T.; Kato, H.; Watanabe, H.; Nakago, S. Twin pregnancy in a 51-year-old woman who underwent autologous cryopreservation at the age of 36 years: Case report. Reprod. Med. Biol. 2016, 15, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.; Venturoli, S.; Damiano, G.; Ciotti, P.; Notarangelo, L.; Paradisi, R.; Moscarini, M.; Ambrosini, G. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod. Biomed. Online 2008, 17, 265–267. [Google Scholar] [CrossRef]
- Do, V.; Catt, S.; Kinder, J.; Walton, S.; Taylor-Robinson, A. Vitrification of In vitro-derived bovine embryos: Targeting enhancement of quality by refining technology and standardising procedures. Reprod. Fertil. Dev. 2019, 31, 837–846. [Google Scholar] [CrossRef]
- Candy, C.J.; Wood, M.J.; Whittingham, D.G.; Merriman, J.A.; Choudhury, N. Cryopreservation of immature mouse oocytes. Hum. Reprod. 1994, 9, 1738–1742. [Google Scholar] [CrossRef]
- Suzuki, T.; Boediono, A.; Takagi, M.; Saha, S.; Sumantri, C. Fertilization and development of frozen-thawed germinal vesicle bovine oocytes by a one-step dilution method In vitro. Cryobiology 1996, 33, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.J.; Wright, G.; Morton, P.C.; Massey, J.B. Birth after cryopreservation of immature oocytes with subsequent In vitro maturation. Fertil. Steril. 1998, 70, 578–579. [Google Scholar] [CrossRef]
- Aono, N.; Abe, Y.; Hara, K.; Sasada, H.; Sato, E.; Yoshida, H. Production of live offspring from mouse germinal vesicle-stage oocytes vitrified by a modified stepwise method, SWEID. Fertil. Steril. 2005, 84, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.C.; Champlin, A.K.; Mobraaten, L.E.; Eppig, J.J. Developmental capacity of mouse oocytes cryopreserved before and after maturation In vitro. J. Reprod. Fertil. 1990, 89, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Van der Elst, J.C.; Nerinckx, S.S.; Van Steirteghem, A.C. Slow and ultrarapid freezing of fully grown germinal vesicle-stage mouse oocytes: Optimization of survival rate outweighed by defective blastocyst formation. J. Assist. Reprod. Genet. 1993, 10, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Aono, N.; Naganuma, T.; Abe, Y.; Hara, K.; Sasada, H.; Sato, E.; Yoshida, H. Successful production of blastocysts following ultrarapid vitrification with step-wise equilibriation of germinal vesicle-stage mouse oocytes. J. Reprod. Dev. 2003, 49, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Frydman, N.; Selva, J.; Bergere, M.; Auroux, M.; Maro, B. Cryopreserved immature mouse oocytes: A chromosomal and spindle study. J. Assist. Reprod. Genet. 1997, 14, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Tan, S.L.; Taketo, T. Beneficial effects of glutathione supplementation during vitrification of mouse oocytes at the germinal vesicle stage on their preimplantation development following maturation and fertilization In vitro. Cryobiology 2017, 76, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Abedpour, N.; Rajaei, F. Vitrification by Cryotop and the Maturation, Fertilization, and Developmental Rates of Mouse Oocytes. Iran. Red Crescent Med. J. 2015, 17, e18172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appeltant, R.; Somfai, T.; Kikuchi, K. Faster, cheaper, defined and efficient vitrification for immature porcine oocytes through modification of exposure time, macromolecule source and temperature. Cryobiology 2018, 85, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Jia, B.; Quan, G.; Xiang, D.; Zhang, B.; Shao, Q.; Hong, Q. Vitrification of porcine immature oocytes: Association of equilibration manners with warming procedures, and permeating cryoprotectants effects under two temperatures. Cryobiology 2017, 75, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Amoushahi, M.; Salehnia, M.; Mowla, S.J. Vitrification of Mouse MII Oocyte Decreases the Mitochondrial DNA Copy Number, TFAM Gene Expression and Mitochondrial Enzyme Activity. J. Reprod. Infertil. 2017, 18, 343–351. [Google Scholar] [PubMed]
- Lei, T.; Guo, N.; Liu, J.Q.; Tan, M.H.; Li, Y.F. Vitrification of In vitro matured oocytes: Effects on meiotic spindle configuration and mitochondrial function. Int, J. Clin. Exp. Pathol. 2014, 7, 1159–1165. [Google Scholar]
- Zhang, Y.; Li, W.; Ma, Y.; Wang, D.; Zhao, X.; Zeng, C.; Zhang, M.; Zeng, X.; Meng, Q.; Zhou, G. Improved development by melatonin treatment after vitrification of mouse metaphase II oocytes. Cryobiology 2016, 73, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Manipalviratn, S.; Tong, Z.B.; Stegmann, B.; Widra, E.; Carter, J.; DeCherney, A. Effect of vitrification and thawing on human oocyte ATP concentration. Fertil. Steril. 2011, 95, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Du, M.; Zhuan, Q.; Luo, Y.; Li, J.; Hou, Y.; Zeng, S.; Zhu, S.; Fu, X. Melatonin rescues the aneuploidy in mice vitrified oocytes by regulating mitochondrial heat product. Cryobiology 2019, 89, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Palomo, M.J.; Albarracín, J.L.; Mogas, T. Vitrification of immature and In vitro matured pig oocytes: Study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology 2004, 49, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.N.; Huang, T.T.; Marikawa, Y. Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes. Biol. Reprod. 2013, 89, 112. [Google Scholar] [CrossRef] [PubMed]
- Nazmara, Z.; Salehnia, M.; HosseinKhani, S. Mitochondrial Distribution and ATP Content of Vitrified, In vitro Matured Mouse Oocytes. Avicenna J. Med. Biotechnol. 2014, 6, 210–217. [Google Scholar] [PubMed]
- Wu, C.; Rui, R.; Dai, J.; Zhang, C.; Ju, S.; Xie, B.; Lu, X.; Zheng, X. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol. Reprod. Dev. 2006, 73, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.S.; Mahesh, Y.U.; Charan, K.V.; Suman, K.; Sekhar, N.; Shivaji, S. Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes. Cryobiology 2012, 64, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Mcguinness, B.E.; Martin, A.; Anna, K.; Gil-Bernabé, A.M.; Wolfgang, H.; Kudo, N.R.; Annelie, W.; Stephen, T.; Christer, H.; Bela, N. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 2009, 19, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Hached, K.; Xie, S.Z.; Buffin, E.; Cladiere, D.; Rachez, C.; Sacras, M.; Sorger, P.K.; Wassmann, K. Mps1 at kinetochores is essential for female mouse meiosis I. Development 2011, 138, 2261–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Dang, C.V.; DeCaprio, J.A. Chapter 17—Control of Cell Division. In Hematology (Seventh Edition); Hoffman, R., Benz, E.J., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 176–185. [Google Scholar]
- Marquardt, J.R.; Fisk, H.A. ARHGEF17 sets the timer for retention of Mps1 at kinetochores. J. Cell Biol. 2016, 212, 615–616. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.C.; Kim, N.H. Spindle assembly checkpoint and its regulators in meiosis. Hum. Reprod. Update 2012, 18, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.L.; Wassmann, K. Multiple Duties for Spindle Assembly Checkpoint Kinases in Meiosis. Front. Cell Dev. Biol. 2017, 5, 109. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Coelho, L.A.; Peres, R.; Amaral, F.G.; Reiter, R.J.; Cipolla-Neto, J. Daily differential expression of melatonin-related genes and clock genes in rat cumulus-oocyte complex: Changes after pinealectomy. J. Pineal Res. 2015, 58, 490–499. [Google Scholar] [CrossRef]
- Kang, J.T.; Koo, O.J.; Kwon, D.K.; Park, H.J.; Jang, G.; Kang, S.K.; Lee, B.C. Effects of melatonin on In vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 2009, 46, 22–28. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Salehi, M.; Masteri Farahani, R.; Dehghani, M.; Abadi, M.; Novin, M.G.; Nourozian, M.; Hosseini, A. The Effect of Melatonin on Maturation, Glutathione Level and Expression of H MGB1 Gene in Brilliant Cresyl Blue (BCB) Stained Immature Oocyte. Cell J. 2014, 15, 294–301. [Google Scholar] [PubMed]
- Rodrigues-Cunha, M.C.; Mesquita, L.G.; Bressan, F.; Collado, M.D.; Balieiro, J.C.; Schwarz, K.R.; de Castro, F.C.; Watanabe, O.Y.; Watanabe, Y.F.; de Alencar Coelho, L.; et al. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequent embryo development. Theriogenology 2016, 86, 1685–1694. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; He, C.; Zhu, K.; Xu, Z.; Ma, T.; Tao, J.; Liu, G. Melatonin protects porcine oocyte In vitro maturation from heat stress. J. Pineal Res. 2015, 59, 365–375. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Maldonado, M.D. Melatonin as an antioxidant: Physiology versus pharmacology. J. Pineal Res. 2005, 39, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, C.J.; Ji, P.Y.; Zhuo, Z.Y.; Tian, X.Z.; Wang, F.; Tan, D.X.; Liu, G.S. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.H.; Leal, C.L.; da Cruz, J.F.; Tan, D.X.; Reiter, R.J. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim. Reprod. Sci. 2014, 145, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhang, C.; Xie, J.; Song, X.; Yin, B.; Liu, Q.; Hu, L.; Hao, H.; Geng, J.; Wang, P. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes In vitro. J. Assist. Reprod. Genet. 2013, 30, 933–938. [Google Scholar] [CrossRef]
- Yan, C.L.; Fu, X.W.; Zhou, G.B.; Zhao, X.M.; Suo, L.; Zhu, S.E. Mitochondrial behaviors in the vitrified mouse oocyte and its parthenogenetic embryo: Effect of Taxol pretreatment and relationship to competence. Fertil. Steril. 2010, 93, 959–966. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Khan, I.; Chowdhury, M.; Song, S.-H.; Mesalam, A.; Zhang, S.; Khalil, A.A.K.; Jung, E.-H.; Kim, J.-B.; Jafri, L.; Mirza, B. Lupeol supplementation improves the developmental competence of bovine embryos In vitro. Theriogenology 2018, 107, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Lan, M.; Zang, X.-W.; Li, Y.-L.; Cui, X.-S.; Kim, N.-H.; Sun, S.-C. Melatonin protects oocytes from MEHP exposure-induced meiosis defects in porcine. Biol. Reprod. 2018, 98, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martín, M.; Yeste, M.; Pericuesta, E.; Morató, R.; Gutiérrez-Adán, A.; Bonet, S. Effects of vitrification on the expression of pluripotency, apoptotic and stress genes in In vitro-produced porcine blastocysts. Reprod. Fertil. Dev. 2015, 27, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Barritt, J.; Moschini, R.M.; Slifkin, R.E.; Copperman, A.B. Optimizing human oocyte cryopreservation for fertility preservation patients: Should we mature then freeze or freeze then mature? Fertil. Steril. 2013, 99, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Brambillasca, F.; Guglielmo, M.C.; Coticchio, G.; Renzini, M.M.; Dal Canto, M.; Fadini, R. The current challenges to efficient immature oocyte cryopreservation. J. Assist. Reprod. Genet. 2013, 30, 1531–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channing, C.P.; Pomerantz, S.H.; Bae, I.H.; Evans, V.W.; Atlas, S.J. Actions of hormones and other factors upon oocyte maturation. Adv. Exp. Med. Biol. 1982, 147, 189–210. [Google Scholar] [PubMed]
- Ma, Y.; Pan, B.; Yang, H.; Qazi, I.H.; Wu, Z.; Zeng, C.; Zhang, M.; Meng, Q.; Zhou, G. Expression of CD9 and CD81 in bovine germinal vesicle oocytes after vitrification followed by In vitro maturation. Cryobiology 2018, 81, 206–209. [Google Scholar] [CrossRef]
- Huang, J.; Ma, Y.; Wei, S.; Pan, B.; Qi, Y.; Hou, Y.; Meng, Q.; Zhou, G.; Han, H. Dynamic changes in the global transcriptome of bovine germinal vesicle oocytes after vitrification followed by In vitro maturation. Reprod. Fertil. Dev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Canesin, H.S.; Brom-de-Luna, J.G.; Choi, Y.H.; Pereira, A.M.; Macedo, G.G.; Hinrichs, K. Vitrification of germinal-vesicle stage equine oocytes: Effect of cryoprotectant exposure time on in-vitro embryo production. Cryobiology 2018, 81, 185–191. [Google Scholar] [CrossRef]
- Gupta, M.K.; Uhm, S.J.; Lee, H.T. Cryopreservation of immature and In vitro matured porcine oocytes by solid surface vitrification. Theriogenology 2007, 67, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.R.; Fisher, P.; Zhu, J.; Choi, I.; Polgar, Z.; Dinnyes, A.; Campbell, K.H. In vitro fertilization of ovine oocytes vitrified by solid surface vitrification at germinal vesicle stage. Cryobiology 2012, 65, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hemadi, M.; Abolhassani, F.; Akbari, M.; Sobhani, A.; Pasbakhsh, P.; Ahrlund-Richter, L.; Modaresi, M.H.; Salehnia, M. Melatonin promotes the cumulus-oocyte complexes quality of vitrified-thawed murine ovaries; with increased mean number of follicles survival and ovary size following heterotopic transplantation. Eur. J. Pharmacol. 2009, 618, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ganji, R.; Nabiuni, M.; Faraji, R. Development of mouse preantral follicle after In vitro culture in a medium containing melatonin. Cell J. 2015, 16, 546–553. [Google Scholar] [PubMed]
- Jiao, G.Z.; Cao, X.Y.; Cui, W.; Lian, H.Y.; Miao, Y.L.; Wu, X.F.; Han, D.; Tan, J.H. Developmental potential of prepubertal mouse oocytes is compromised due mainly to their impaired synthesis of glutathione. PloS ONE 2013, 8, e58018. [Google Scholar] [CrossRef] [PubMed]
- Luz, H.K.M.; Wanderley, L.S.; Faustino, L.R.; da Silva, C.M.G.; de Figueiredo, J.R.; Rodrigues, A.P.R. Role of Antioxidants Agents in Germ Cells and Embryos Cryopreservation. Acta Sci. Vet. 2011, 39, 956. [Google Scholar]
- De Matos, D.G.; Furnus, C.C. The importance of having high glutathione (GSH) level after bovine In vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology 2000, 53, 761–771. [Google Scholar] [CrossRef]
- Hardeland, R. Atioxidative protection by melatonin. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Leon, J.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9. [Google Scholar] [CrossRef]
- He, B.; Yin, C.; Gong, Y.; Liu, J.; Guo, H.; Zhao, R. Melatonin-induced increase of lipid droplets accumulation and In vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J. Cell. Physiol. 2018, 233, 302–312. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Novin, M.G.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin modifies histone acetylation during In vitro maturation of mouse oocytes. Cell J. (Yakhteh) 2018, 20, 244. [Google Scholar]
- Dai, J.; Wu, C.; Muneri, C.W.; Niu, Y.; Zhang, S.; Rui, R.; Zhang, D. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 2015, 71, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M.; Rottenberg, H. Relation between the gradient of the ATP/ADP ratio and the membrane potential across the mitochondrial membrane. Eur. J. Biochem. 1977, 73, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zander-Fox, D.; Cashman, K.S.; Lane, M. The presence of 1 mM glycine in vitrification solutions protects oocyte mitochondrial homeostasis and improves blastocyst development. J. Assist. Reprod. Genet. 2013, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011, 11, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.-J.; Peng, T.-I.; Yu, P.-Z.; Jou, S.-B.; Reiter, R.J.; Chen, J.-Y.; Wu, H.-Y.; Chen, C.-C.; Hsu, L.-F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2010, 43, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Fuller, B.J. Cryopreservation of human oocytes: A review of current problems and perspectives. Hum. Reprod. Update 1996, 2, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.J.; Braude, P.R.; Johnson, M.H.; Cant, A.; Currie, J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil. Steril. 1990, 54, 102–108. [Google Scholar] [CrossRef]
- Ko, C.S.; Ding, D.C.; Chu, T.W.; Chu, Y.N.; Chen, I.C.; Chen, W.H.; Wu, G.J. Changes to the meiotic spindle and zona pellucida of mature mouse oocytes following different cryopreservation methods. Anim. Reprod. Sci. 2008, 105, 272–282. [Google Scholar] [CrossRef] [PubMed]
- McNally, F.J. Mechanisms of spindle positioning. J. Cell Biol. 2013, 200, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Wang, H.; Li, L.; Li, X.; Ge, J.; Reiter, R.J.; Wang, Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J. Pineal Res. 2017, 63. [Google Scholar] [CrossRef]
- Zhou, G.B.; Zeng, Y.; Meng, Q.G.; Liu, Y.; Dai, Y.P.; Zhu, S.E.; Bunch, T.D.; Hou, Y.P. Decreased expression of CD9 in bovine oocytes after cryopreservation and the relationship to fertilization capacity. Mol. Reprod. Dev. 2013, 80, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, L.; Zhao, Q.; Gao, Z.; Xu, X. Lycium barbarum polysaccharide improves the development of mouse oocytes vitrified at the germinal vesicle stage. Cryobiology 2018, 85, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, X.L.; Guo, X.F.; Zhang, F.; Pei, X.Z.; Li, X.X.; Han, W.X.; Li, Y.H. Effect of liquid helium vitrification on the ultrastructure and related gene expression of mature bovine oocytes after vitrifying at immature stage. Theriogenology 2017, 87, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Khodabandeh, Z.; Nori Mogahi, M.H. Comparison of The Effects of Vitrification on Gene Expression of Mature Mouse Oocytes Using Cryotop and Open Pulled Straw. Int. J. Fertil. Steril. 2018, 12, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.-A.; Forcada, F.; Vázquez, M.-I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Pérez-Pe, R.; Casao, A. Role of melatonin on embryo viability in sheep. Reprod. Fertil. Dev. 2019, 31, 82–92. [Google Scholar] [CrossRef]
- Casao, A.; Abecia, J.; Pérez, J.C.; Blanco, T.M.; Vázquez, M.; Forcada, F. The effects of melatonin on In vitro oocyte competence and embryo development in sheep. Span. J. Agric. Res. 2010, 8, 35–41. [Google Scholar] [CrossRef]






| Gene | Accession ID | Primer Seq (5′-3′) | Product Length (bp) | Tm (°C) |
|---|---|---|---|---|
| Mad2 | NM_001355624.1 | F: GAGAGCAAGGCATCACCCTG | 103 | 60.2 |
| R: TCCGACGGATAAATGCCACG | ||||
| BubR1 | NM_009773.3 | F: TCCAGGAAGGGATTGAACGC | 154 | 60.2 |
| R: AGCGAGCTTCTCTGTGGTTC | ||||
| Mps1 | NM_001110265.1 | F: CCATGGGAACGCAAGAGCTA | 284 | 60.2 |
| R: GCTCTTCCCGGTACCTTGGTC | ||||
| Mad1 | NM_001359025.1 | F: CCTGGGATCCTACGACAGTG | 105 | 60.2 |
| R: CTGTGGGCATGTACCTTCTGA | ||||
| Gapdh | NM_008084.3 | F: CATGGCCTTCCGTGTTCCTA | 104 | 60.2 |
| R:GCCTGCTTACCACCTTCTT |
| Groups | Treated with Melatonin(mol/L) | No. of GV Oocytes Vitrified | No. of GV Oocytes Recovered | No. of GV Oocytes with Normal Morphology | No. of GV Oocytes that Matured to | ||
|---|---|---|---|---|---|---|---|
| GVBD Stage at 5 h.p.i. (%) | MI Stage at 9 h.p.i. (%) | MII Stage at 13 h.p.i. (%) | |||||
| F | 0 | 54 | 51(95.45 ± 0.04) a | 47(85.76 ± 0.05) a | 44(81.21 ± 0.01) a | ||
| V | 0 | 67 | 60 | 56 | 46(85.93 ± 0.06) b,e | 41(74.07 ± 0.04) b,e,f | 35(61.30 ± 0.02) c |
| V + M | 10−9 | 65 | 59 | 57 | 26(45.71 ± 0.01) c | 19(34.09 ± 0.05) c | 11(19.39 ± 0.01) f |
| V + M | 10−7 | 65 | 61 | 52 | 47(89.97 ± 0.04) a,b | 41(78.52 ± 0.06) a,e | 38(72.67 ± 0.06) b |
| V + M | 10−5 | 63 | 56 | 52 | 41(78.75 ± 0.03) e | 37(71.25 ± 0.03) b,f | 20(37.92 ± 0.06) d |
| V + M | 10−3 | 62 | 56 | 50 | 30(59.98 ± 0.01) d | 26(52.09 ± 0.02) d | 15(29.80 ± 0.03) e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Pan, B.; Qazi, I.H.; Yang, H.; Guo, S.; Yang, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Han, H.; et al. Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells 2019, 8, 1009. https://doi.org/10.3390/cells8091009
Wu Z, Pan B, Qazi IH, Yang H, Guo S, Yang J, Zhang Y, Zeng C, Zhang M, Han H, et al. Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells. 2019; 8(9):1009. https://doi.org/10.3390/cells8091009
Chicago/Turabian StyleWu, Zhenzheng, Bo Pan, Izhar Hyder Qazi, Haoxuan Yang, Shichao Guo, Jingyu Yang, Yan Zhang, Changjun Zeng, Ming Zhang, Hongbing Han, and et al. 2019. "Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes" Cells 8, no. 9: 1009. https://doi.org/10.3390/cells8091009
APA StyleWu, Z., Pan, B., Qazi, I. H., Yang, H., Guo, S., Yang, J., Zhang, Y., Zeng, C., Zhang, M., Han, H., Meng, Q., & Zhou, G. (2019). Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells, 8(9), 1009. https://doi.org/10.3390/cells8091009