Physiology, Pathology and Regeneration of Salivary Glands
Abstract
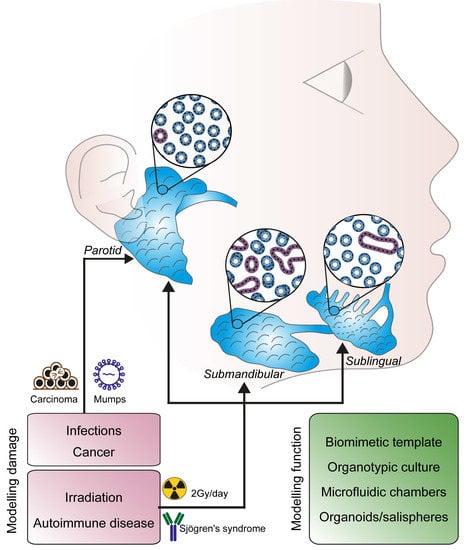
:1. Introduction
2. Salivary Gland Anatomy and Morphogenesis
2.1. Morphogenesis of the Salivary Glands
2.2. Histological and Anatomical Features
2.3. Innervation and Trophic Support
3. Chemistry of Secretion and Functions of Salivary Glands
3.1. Chemical Composition of Saliva
3.2. Protein Components of Saliva and Degranulation
3.3. Mechanical Control of Saliva Secretion
4. Salivary Gland Disorders
4.1. Tumours
4.2. Primary Sjögren’s Syndrome
4.3. Post-Irradiation Syndrome
4.4. Infections
5. Molecular Pathways Involved in Salivary Gland Functionality
6. Regenerative Medicine and Salivary Glands Stem Cells
Stem Cells Therapies
7. Perspective and Future Directions
7.1. Biomimetic Models and Organ Cultures
7.2. Live Imaging of Functional Glands
7.3. Microfluidic Chambers
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| PG | Parotid gland |
| SL | Sublingual gland |
| SMG | Submandibular gland |
| EGF | Epidermal growth factor |
| FGF | Fibroblast growth factor |
| NGF | Nerve growth factor |
| TGF- α | Transforming growth factor- α |
| GCT | Granular convoluted tubules |
| PSG | Parasympathetic submandibular ganglion |
| Krt | Keratin |
| ER | Endoplasmatic reticulum |
| AQP5 | Aquaporin 5 |
| SNAP | Soluble NSF attachment proteins |
| SNAREs | SNAP REceptor |
| cAMP | Cyclic adenosine monophosphate |
| CREB | cAMP-responsive element binding |
| CRTC1 | CREB Regulated Transcription Coactivator 1 |
| MAML2 | Mastermind-like 2 |
| pSS | Primary Sjögren’s syndrome |
| TNF | Tumor necrosis factor |
| BAFF | B-cell activating factor |
| APRIL | A proliferation-inducing ligand |
| HIV | Human immunodeficiency viruses |
| GDNF | Glial cell line-derived neurotrophic factor |
| Int | Integrin |
| SOX | SRY-related HMG-box |
| MUC | Mucin |
References
- Amano, O.; Mizobe, K.; Bando, Y.; Sakiyama, K. Anatomy and histology of rodent and human major salivary glands: Overview of the Japan salivary gland society-sponsored workshop. Acta Histochem. Cytochem. 2012, 45, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Dobrosielski-Vergona, K. Biology of the Salivary Glands; CRC Press, Taylor & Francis Group: Abingdon, UK, 1993; ISBN 978-0849388477. [Google Scholar]
- Korsrud, F.R.; Brandtzaeg, P. Quantitative immunohistochemistry of immunoglobulin- and J-chain-producing cells in human parotid and submandibular salivary glands. Immunology 1980, 39, 129–140. [Google Scholar] [PubMed]
- Smith, D.J.; Taubman, M.A.; King, W.F. Immunological features of minor salivary gland saliva. J. Clin. Immunol. 1987, 7, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Treuting, P.M.; Dintzis, S.M.; Frevert, C.W.; Liggitt, D.; Liggitt, H.D.; Montine, K.S. Comparative Anatomy and Histology: A Mouse and Human Atlas (Expert Consult); Elsevier Inc.: UK, 2012; Available online: https://books.google.ch/books?id=Bqn23_270Q8C (accessed on 3 August 2019).
- Kondo, Y.; Nakamoto, T.; Jaramillo, Y.; Choi, S.; Catalan, M.A.; Melvin, J.E. Functional differences in the acinar cells of the murine major salivary glands. J. Dent. Res. 2015, 94, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, R. Role of saliva in the maintenance of taste sensitivity. Crit. Rev. Oral Biol. Med. 2000, 11, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Ono Minagi, H.; Sarper, S.E.; Kurosaka, H.; Kuremoto, K.I.; Taniuchi, I.; Sakai, T.; Yamashiro, T. Runx1 mediates the development of the granular convoluted tubules in the submandibular glands. PLoS ONE 2017, 12, e0184395. [Google Scholar] [CrossRef]
- Mori, M.; Sumitomo, S.; Shrestha, P.; Tanaka, S.; Takai, Y.; Shikimori, M. Multifunctional roles of growth factors or biologically active peptides in salivary glands and saliva. Oral Med. Pathol. 2008, 12, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Rojo, L.; Granchi, Z.; Graf, D.; Mitsiadis, T.A. Stem Cell Fate Determination during Development and Regeneration of Ectodermal Organs. Front. Physiol. 2012, 3, 107. [Google Scholar] [CrossRef] [Green Version]
- Gervais, E.M.; Sequeira, S.J.; Wang, W.; Abraham, S.; Kim, J.H.; Leonard, D.; DeSantis, K.A.; Larsen, M. Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis 2016, 12, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Chatzeli, L.; Gaete, M.; Tucker, A.S. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 2017, 144, 2294–2305. [Google Scholar] [CrossRef]
- Carlson, B.M. Human Embryology and Developmental Biology E-Book; Elsevier Health Sciences: UK, 2008; Available online: https://books.google.ch/books?id=xnK5_R_jeboC (accessed on 3 August 2019).
- Carlson, B.M. Human Embryology and Developmental Biology; Mosby/Elsevier: Philadelphia, PA, USA, 2009; Available online: http://www.clinicalkey.com.au/dura/browse/bookChapter/3-s2.0-C20090336673 (accessed on 28 July 2019).
- Pagella, P.; Jiménez-Rojo, L.; Mitsiadis, T.A. Roles of innervation in developing and regenerating orofacial tissues. Cell Mol. Life Sci. 2014, 71, 2241–2251. [Google Scholar] [CrossRef] [Green Version]
- Coughlin, M.D. Early development of parasympathetic nerves in the mouse submandibular gland. Dev. Biol. 1975, 43, 123–139. [Google Scholar] [CrossRef]
- Patel, V.N.; Rebustini, I.T.; Hoffman, M.P. Salivary gland branching morphogenesis. Differentiation 2006, 74, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Menko, A.S.; Khalil, S.; Rebustini, I.; Hoffman, M.P.; Kreidberg, J.A.; Kukuruzinska, M.A. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: Insights into the formation of acinar and ductal structures. Dev. Dyn. 2008, 237, 3128–3141. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.R.; Nelson, D.A.; DeSantis, K.A.; Morrissey, J.M.; Larsen, M. Endothelial cell regulation of salivary gland epithelial patterning. Development 2017, 144, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef]
- Iaizzo, P.A. Introduction to Neurophysiology. In Neural Eng.; He, B., Ed.; Springer: Boston, MA, USA, 2013; pp. 1–86. [Google Scholar] [CrossRef]
- Lundberg, A. Electrophysiology of salivary glands. Physiol Rev. 1958, 38, 21–40. [Google Scholar] [CrossRef]
- Alm, P. Adrenergic and cholinergic nerves of bovine, guinea pig and hamster salivary glands. A light and electron microscopic study. Z. Zellforsch. Mikrosk. Anat. 1973, 138, 407–420. [Google Scholar] [CrossRef]
- Garrett, J.R. Neuro-Effector Sites in Salivary Glands. In Oral Physiology; Elsevier: UK, 1972; pp. 83–97. [Google Scholar] [CrossRef]
- Garret, J.R.; Kidd, A. Effects of autonomic nerve stimulation on submandibular acini and saliva in cats [proceedings]. J. Physiol. 1976, 263, 198P–199P. [Google Scholar]
- Patel, V.N.; Hoffman, M.P. Salivary gland development: A template for regeneration. Semin. Cell Dev. Biol. 2014, 25–26, 52–60. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010, 329, 1645–1647. [Google Scholar] [CrossRef]
- Makita, T.; Sucov, H.M.; Gariepy, C.E.; Yanagisawa, M.; Ginty, D.D. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature 2008, 452, 759–763. [Google Scholar] [CrossRef] [Green Version]
- Ventimiglia, M.S.; Rodriguez, M.R.; Morales, V.P.; Elverdin, J.C.; Perazzo, J.C.; Castañ, M.M.; Davio, C.A.; Vatta, M.S.; Bianciotti, L.G. Endothelins participate in the central and peripheral regulation of submandibular gland secretion in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R109–R120. [Google Scholar] [CrossRef] [Green Version]
- Glebova, N.O.; Ginty, D.D. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J. Neurosci. 2004, 24, 743–751. [Google Scholar] [CrossRef]
- Levi-Montalcini, R.; Angeletti, P.U. Nerve growth factor. Physiol. Rev. 1968, 48, 534–569. [Google Scholar] [CrossRef]
- Murphy, R.A.; Saide, J.D.; Blanchard, M.H.; Young, M. Nerve growth factor in mouse serum and saliva: Role of the submandibular gland. Proc. Natl. Acad. Sci. USA 1977, 74, 2330–2333. [Google Scholar] [CrossRef]
- Garrett, J.R.; Kidd, A. The innervation of salivary glands as revealed by morphological methods. Microsc. Res. Tech. 1993, 26, 75–91. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Nanci, A. Ten Cate’s Oral Histology; Elsevier Inc.: UK, 2012; Available online: https://books.google.ch/books?isbn=0323242073 (accessed on 3 August 2019).
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2016, 17, 166. [Google Scholar] [CrossRef]
- Takuma, T.; Tagaya, M.; Ichida, T. Evidence for the putative docking/fusion complex of exocytosis in parotid acinar cells. FEBS Lett. 1997, 404, 34–36. [Google Scholar] [CrossRef] [Green Version]
- Fujita-Yoshigaki, J.; Dohke, Y.; Hara-Yokoyama, M.; Kamata, Y.; Kozaki, S.; Furuyama, S.; Sugiya, H. Vesicle-associated membrane protein 2 is essential for cAMP-regulated exocytosis in rat parotid acinar cells. The inhibition of cAMP-dependent amylase release by botulinum neurotoxin B. J. Biol. Chem. 1996, 271, 13130–13134. [Google Scholar] [CrossRef]
- Garrett, J.R.; Thulin, A. Changes in parotid acinar cells accompanying salivary secretion in rats on sympathetic or parasympathetic nerve stimulation. Cell Tissue Res. 1975, 159, 179–193. [Google Scholar] [CrossRef]
- Segawa, A.; Terakawa, S.; Yamashina, S.; Hopkins, C.R. Exocytosis in living salivary glands: Direct visualization by video-enhanced microscopy and confocal laser microscopy. Eur. J. Cell Biol. 1991, 54, 322–330. [Google Scholar]
- Roukema, P.A.; Oderkerk, C.H.; Salkinoga-Salonen, M.S. The murine sublingual and submandibular mucins. Their isolation and characterization. Biochim. Biophys. Acta 1976, 428, 432–440. [Google Scholar] [CrossRef]
- Kim, S.K.; Nasjleti, C.E.; Han, S.S. The secretion processes in mucous and serous secretory cells of the rat sublingual gland. J. Ultrastruct. Res. 1972, 38, 371–389. [Google Scholar] [CrossRef]
- Garrett, J.R. The proper role of nerves in salivary secretion: A review. J. Dent. Res. 1987, 66, 387–397. [Google Scholar] [CrossRef]
- Vreugdenhil, A.P.; Nieuw Amerongen, A.V.; De Lange, G.L.; Roukema, P.A. Localization of amylase and mucins in the major salivary glands of the mouse. Histochem. J. 1982, 14, 767–780. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Thomas, U.; Pellegrini, A. A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 2001, 276, 43767–43774. [Google Scholar] [CrossRef]
- Laible, N.J.; Germaine, G.R. Bactericidal activity of human lysozyme, muramidase-inactive lysozyme, and cationic polypeptides against Streptococcus sanguis and Streptococcus faecalis: Inhibition by chitin oligosaccharides. Infect. Immun. 1985, 48, 720–728. [Google Scholar]
- Sava, G.; Benetti, A.; Ceschia, V.; Pacor, S. Lysozyme and cancer: Role of exogenous lysozyme as anticancer agent (review). Anticancer Res. 1989, 9, 583–591. [Google Scholar]
- Sun, H.; Chen, Y.; Zou, X.; Li, Q.; Li, H.; Shu, Y.; Li, X.; Li, W.; Han, L.; Ge, C. Salivary Secretory Immunoglobulin (SIgA) and Lysozyme in Malignant Tumor Patients. Biomed. Res. Int. 2016, 2016, 8701423. [Google Scholar] [CrossRef]
- Noble, R.E. Salivary alpha-amylase and lysozyme levels: A non-invasive technique for measuring parotid vs submandibular/sublingual gland activity. J. Oral Sci. 2000, 42, 83–86. [Google Scholar] [CrossRef]
- Veerman, E.C.; Van den Keybus, P.A.; Vissink, A.; Nieuw Amerongen, A.V. Human glandular salivas: Their separate collection and analysis. Eur. J. Oral Sci. 1996, 104, 346–352. [Google Scholar] [CrossRef]
- Garrett, J.R.; Emmelin, N. Activities of salivary myoepithelial cells: A review. Med. Biol. 1979, 57, 1–28. [Google Scholar]
- Emmelin, N.; Garrett, J.R.; Gjörstrup, P. Supporting effects of myoepithelial cells in submandibular glands of dogs when acting against increased intraluminal pressure. J. Physiol. 1977, 268, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, B.; Mahabob, M.N. Mucocele: An unusual presentation of the minor salivary gland lesion. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. S2), S180–S182. [Google Scholar] [CrossRef]
- Pinkston, J.A.; Cole, P. Incidence rates of salivary gland tumors: Results from a population-based study. Otolaryngol. Head Neck Surg. 1999, 120, 834–840. [Google Scholar] [CrossRef]
- Stenner, M.; Klussmann, J.P. Current update on established and novel biomarkers in salivary gland carcinoma pathology and the molecular pathways involved. Eur. Arch. Otorhinolaryngol. 2009, 266, 333–341. [Google Scholar] [CrossRef]
- Alvi, S.; Chudek, D.; Limaiem, F. Cancer, Parotid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538340/ (accessed on 28 July 2019).
- Yan, K.; Yesensky, J.; Hasina, R.; Agrawal, N. Genomics of mucoepidermoid and adenoid cystic carcinomas. Laryngoscope Investig. Otolaryngol. 2018, 3, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Emmerson, E.; Knox, S.M. Salivary gland stem cells: A review of development, regeneration and cancer. Genesis 2018, 56, e23211. [Google Scholar] [CrossRef]
- Manvikar, V.; Ramulu, S.; Ravishanker, S.T.; Chakravarthy, C. Squamous cell carcinoma of submandibular salivary gland: A rare case report. J. Oral Maxillofac. Pathol. 2014, 18, 299–302. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Mendenhall, C.M.; Werning, J.W.; Malyapa, R.S.; Mendenhall, N.P. Salivary gland pleomorphic adenoma. Am. J. Clin. Oncol. 2008, 31, 95–99. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Gu, Y.; Hu, C.; Li, J.L.; Lin, S.; Shen, H.; Cao, C.; Gao, R.; Li, J.; et al. Aberrantly activated AREG-EGFR signaling is required for the growth and survival of CRTC1-MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene 2014, 33, 3869–3877. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.-L.; Chen, Z.; Griffin, J.D.; Wu, L. Gene expression profiling analysis of CRTC1-MAML2 fusion oncogene-induced transcriptional program in human mucoepidermoid carcinoma cells. BMC Cancer 2015, 15, 803. [Google Scholar] [CrossRef]
- Tonon, G.; Modi, S.; Wu, L.; Kubo, A.; Coxon, A.B.; Komiya, T.; O’Neil, K.; Stover, K.; El-Naggar, A.; Griffin, J.D.; et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat. Genet. 2003, 33, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Behboudi, A.; Enlund, F.; Winnes, M.; Andrén, Y.; Nordkvist, A.; Leivo, I.; Flaberg, E.; Szekely, L.; Mäkitie, A. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer 2006, 45, 470–481. [Google Scholar] [CrossRef]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef]
- Rettig, E.M.; Talbot, C.C.; Sausen, M.; Jones, S.; Bishop, J.A.; Wood, L.D.; Tokheim, C.; Niknafs, N.; Karchin, R.; Fertig, E.J.; et al. Whole-Genome Sequencing of Salivary Gland Adenoid Cystic Carcinoma. Cancer Prev. Res. 2016, 9, 265–274. [Google Scholar] [CrossRef]
- Chen, W.; Cao, G.; Yuan, X.; Zhang, X.; Zhang, Q.; Zhu, Y.; Dong, Z.; Zhang, S. Notch-1 knockdown suppresses proliferation, migration and metastasis of salivary adenoid cystic carcinoma cells. J. Transl. Med. 2015, 13, 167. [Google Scholar] [CrossRef]
- Qu, J.; Song, M.; Xie, J.; Huang, X.Y.; Hu, X.M.; Gan, R.H.; Zhao, Y.; Lin, L.S.; Chen, J.; Lin, X.; et al. Notch2 signaling contributes to cell growth, invasion, and migration in salivary adenoid cystic carcinoma. Mol. Cell Biochem. 2016, 411, 135–141. [Google Scholar] [CrossRef]
- Groom, J.; Kalled, S.L.; Cutler, A.H.; Olson, C.; Woodcock, S.A.; Schneider, P.; Tschopp, J.; Cachero, T.G.; Batten, M.; Wheway, J.; et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J. Clin. Investig. 2002, 109, 59–68. [Google Scholar] [CrossRef]
- Mackay, F.; Browning, J.L. BAFF: A fundamental survival factor for B cells. Nat. Rev. Immunol. 2002, 2, 465–475. [Google Scholar] [CrossRef]
- He, B.; Chadburn, A.; Jou, E.; Schattner, E.J.; Knowles, D.M.; Cerutti, A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J. Immunol. 2004, 172, 3268–3279. [Google Scholar] [CrossRef]
- Mariette, X.; Seror, R.; Quartuccio, L.; Baron, G.; Salvin, S.; Fabris, M.; Desmoulins, F.; Nocturne, G.; Ravaud, P.; De Vita, S. Efficacy and safety of belimumab in primary Sjögren’s syndrome: Results of the BELISS open-label phase II study. Ann. Rheum. Dis. 2015, 74, 526–531. [Google Scholar] [CrossRef]
- De Vita, S.; Quartuccio, L.; Salvin, S.; Picco, L.; Scott, C.A.; Rupolo, M.; Fabris, M. Sequential therapy with belimumab followed by rituximab in Sjögren’s syndrome associated with B-cell lymphoproliferation and overexpression of BAFF: Evidence for long-term efficacy. Clin. Exp. Rheumatol. 2014, 32, 490–494. [Google Scholar]
- Steinfeld, S.D.; Tant, L.; Burmester, G.R.; Teoh, N.K.; Wegener, W.A.; Goldenberg, D.M.; Pradier, O. Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren’s syndrome: An open-label phase I/II study. Arthritis Res. Ther. 2006, 8, R129. [Google Scholar] [CrossRef]
- Dass, S.; Bowman, S.J.; Vital, E.M.; Ikeda, K.; Pease, C.T.; Hamburger, J.; Richards, A.; Rauz, S.; Emery, P. Reduction of fatigue in Sjögren syndrome with rituximab: Results of a randomised, double-blind, placebo-controlled pilot study. Ann. Rheum. Dis. 2008, 67, 1541–1544. [Google Scholar] [CrossRef]
- Devauchelle-Pensec, V.; Mariette, X.; Jousse-Joulin, S.; Berthelot, J.M.; Perdriger, A.; Puéchal, X.; Le Guern, V.; Sibilia, J.; Gottenberg, J.E.; Chiche, L.; et al. Treatment of primary Sjögren syndrome with rituximab: A randomized trial. Ann. Intern. Med. 2014, 160, 233–242. [Google Scholar] [CrossRef]
- Vissink, A.; Mitchell, J.B.; Baum, B.J.; Limesand, K.H.; Jensen, S.B.; Fox, P.C.; Elting, L.S.; Langendijk, J.A.; Coppes, R.P.; Reyland, M.E.; et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 983–991. [Google Scholar] [CrossRef]
- Burlage, F.R.; Coppes, R.P.; Meertens, H.; Stokman, M.A.; Vissink, A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother. Oncol. 2001, 61, 271–274. [Google Scholar] [CrossRef]
- Pinna, R.; Campus, G.; Cumbo, E.; Mura, I.; Milia, E. Xerostomia induced by radiotherapy: An overview of the physiopathology, clinical evidence, and management of the oral damage. Ther. Clin. Risk Manag. 2015, 11, 171–188. [Google Scholar] [CrossRef]
- Visvanathan, V.; Nix, P. Managing the patient presenting with xerostomia: A review. Int. J. Clin. Pract. 2010, 64, 404–407. [Google Scholar] [CrossRef]
- Aframian, D.J.; Mizrahi, B.; Granot, I.; Domb, A.J. Evaluation of a mucoadhesive lipid-based bioerodable tablet compared with Biotène mouthwash for dry mouth relief—A pilot study. Quintessence Int. 2010, 41, e36–e42. [Google Scholar]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2015, 11, 45–51. [Google Scholar] [CrossRef]
- Wilson, K.F.; Meier, J.D.; Ward, P.D. Salivary gland disorders. Am. Fam. Phys. 2014, 89, 882–888. [Google Scholar]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Kampinga, H.H.; De Haan, G.; Coppes, R.P. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008, 26, 2595–2601. [Google Scholar] [CrossRef]
- Häärä, O.; Fujimori, S.; Schmidt-Ullrich, R.; Hartmann, C.; Thesleff, I.; Mikkola, M.L. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development 2011, 138, 2681–2691. [Google Scholar] [CrossRef] [Green Version]
- Hai, B.; Yang, Z.; Millar, S.E.; Choi, Y.S.; Taketo, M.M.; Nagy, A.; Liu, F. Wnt/β-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev. 2010, 19, 1793–1801. [Google Scholar] [CrossRef]
- Jaskoll, T.; Leo, T.; Witcher, D.; Ormestad, M.; Astorga, J.; Bringas, P., Jr.; Carlsson, P.; Melnick, M. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev. Dyn. 2004, 229, 722–732. [Google Scholar] [CrossRef]
- Hai, B.; Qin, L.; Yang, Z.; Zhao, Q.; Shangguan, L.; Ti, X.; Zhao, Y.; Kim, S.; Rangaraj, D.; Liu, F. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin. Cancer Res. 2014, 20, 140–150. [Google Scholar] [CrossRef]
- Haberman, A.S.; Isaac, D.D.; Andrew, D.J. Specification of cell fates within the salivary gland primordium. Dev. Biol. 2003, 258, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Mitsiadis, T.A.; Henrique, D.; Thesleff, I.; Lendahl, U. Mouse Serrate-1 (Jagged-1): Expression in the developing tooth is regulated by epithelial-mesenchymal interactions and fibroblast growth factor-4. Development 1997, 124, 1473–1483. [Google Scholar]
- Dang, H.; Lin, A.L.; Zhang, B.; Zhang, H.-M.; Katz, M.S.; Yeh, C.-K. Role for Notch signaling in salivary acinar cell growth and differentiation. Dev. Dyn. 2009, 238, 724–731. [Google Scholar] [CrossRef]
- Jhappan, C.; Gallahan, D.; Stahle, C.; Chu, E.; Smith, G.H.; Merlino, G.; Callahan, R. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1996, 6, 345–355. [Google Scholar] [CrossRef]
- Rossi, J.; Luukko, K.; Poteryaev, D.; Laurikainen, A.; Sun, Y.F.; Laakso, T.; Eerikäinen, S.; Tuominen, R.; Lakso, M.; Rauvala, H.; et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron 1999, 22, 243–252. [Google Scholar] [CrossRef]
- Cohen, S. Origins of growth factors: NGF and EGF. Ann. N. Y. Acad. Sci. 2004, 1038, 98–102. [Google Scholar] [CrossRef]
- Ghasemlou, N.; Krol, K.M.; Macdonald, D.R.; Kawaja, M.D. Comparison of target innervation by sympathetic axons in adult wild type and heterozygous mice for nerve growth factor or its receptor trkA. J. Pineal Res. 2004, 37, 230–240. [Google Scholar] [CrossRef]
- Schenck, K.; Schreurs, O.; Hayashi, K.; Helgeland, K. The Role of Nerve Growth Factor (NGF) and Its Precursor Forms in Oral Wound Healing. Int. J. Mol. Sci. 2017, 18, 386. [Google Scholar] [CrossRef]
- Søland, T.M.; Brusevold, I.J.; Koppang, H.S.; Schenck, K.; Bryne, M. Nerve growth factor receptor (p75 NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology 2008, 53, 62–72. [Google Scholar] [CrossRef]
- Kadoya, Y.; Kadoya, K.; Durbeej, M.; Holmvall, K.; Sorokin, L.; Ekblom, P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J. Cell Biol. 1995, 129, 521–534. [Google Scholar] [CrossRef]
- Menko, A.S.; Kreidberg, J.A.; Ryan, T.T.; Van Bockstaele, E.; Kukuruzinska, M.A. Loss of alpha3beta1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev. Dyn. 2001, 220, 337–349. [Google Scholar] [CrossRef]
- Rebustini, I.T.; Patel, V.N.; Stewart, J.S.; Layvey, A.; Georges-Labouesse, E.; Miner, J.H.; Hoffman, M.P. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev. Biol. 2007, 308, 15–29. [Google Scholar] [CrossRef]
- Hecht, D.; Jung, D.; Prabhu, V.V.; Munson, P.J.; Hoffman, M.P.; Kleinman, H.K. Metallothionein promotes laminin-1-induced acinar differentiation in vitro and reduces tumor growth in vivo. Cancer Res. 2002, 62, 5370–5374. [Google Scholar]
- Sato, A.; Okumura, K.; Matsumoto, S.; Hattori, K.; Hattori, S.; Shinohara, M.; Endo, F. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells 2007, 9, 191–205. [Google Scholar] [CrossRef]
- Laine, M.; Virtanen, I.; Salo, T.; Konttinen, Y.T. Segment-specific but pathologic laminin isoform profiles in human labial salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum. 2004, 50, 3968–3973. [Google Scholar] [CrossRef]
- Lemercier, C.; To, R.Q.; Swanson, B.J.; Lyons, G.E.; Konieczny, S.F. Mist1: A novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Dev. Biol. 1997, 182, 101–113. [Google Scholar] [CrossRef]
- Yoshida, S.; Ohbo, K.; Takakura, A.; Takebayashi, H.; Okada, T.; Abe, K.; Nabeshima, Y. Sgn1, a basic helix-loop-helix transcription factor delineates the salivary gland duct cell lineage in mice. Dev. Biol. 2001, 240, 517–530. [Google Scholar] [CrossRef]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Berthoin, L.; Cruz-Pacheco, N.; Nathan, S.; Mattingly, A.J.; Chang, J.L.; Ryan, W.R.; Tward, A.D.; Knox, S.M. Salivary glands regenerate after radiation injury through SOX2-mediated secretory cell replacement. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Feng, J.; van der Zwaag, M.; Stokman, M.A.; van Os, R.; Coppes, R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother. Oncol. 2009, 92, 466–471. [Google Scholar] [CrossRef]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef]
- Hisatomi, Y.; Okumura, K.; Nakamura, K.; Matsumoto, S.; Satoh, A.; Nagano, K.; Yamamoto, T.; Endo, F. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology 2004, 39, 667–675. [Google Scholar] [CrossRef]
- Bullard, T.; Koek, L.; Roztocil, E.; Kingsley, P.D.; Mirels, L.; Ovitt, C.E. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev. Biol. 2008, 320, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Arany, S.; Catalán, M.A.; Roztocil, E.; Ovitt, C.E. Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration. Dev. Biol. 2011, 353, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Rugel-Stahl, A.; Elliott, M.E.; Ovitt, C.E. Ascl3 marks adult progenitor cells of the mouse salivary gland. Stem Cell Res. 2012, 8, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Schröck, A.; Bode, M.; Göke, F.J.M.; Bareiss, P.M.; Schairer, R.; Wang, H.; Weichert, W.; Franzen, A.; Kirsten, R.; van Bremen, T.; et al. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis 2014, 35, 1636–1642. [Google Scholar] [CrossRef]
- Matheu, A.; Collado, M.; Wise, C.; Manterola, L.; Cekaite, L.; Tye, A.J.; Canamero, M.; Bujanda, L.; Schedl, A.; Cheah, K.S.; et al. Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 2012, 72, 1301–1315. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Panaccione, A.; Nonaka, D.; Prasad, M.L.; Boyd, K.L.; Brown, B.; Guo, Y.; Sewell, A.; Yarbrough, W.G. Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br. J. Cancer 2013, 109, 444–451. [Google Scholar] [CrossRef]
- Schoenhals, M.; Kassambara, A.; De Vos, J.; Hose, D.; Moreaux, J.; Klein, B. Embryonic stem cell markers expression in cancers. Biochem. Biophys. Res. Commun. 2009, 383, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Redman, R.S. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech. Histochem. 2008, 83, 103–130. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.; Manzella, K.; Baker, O.J. Current cell models for bioengineering a salivary gland: A mini-review of emerging technologies. Oral Dis. 2013, 19, 236–244. [Google Scholar] [CrossRef]
- Aframian, D.J.; Tran, S.D.; Cukierman, E.; Yamada, K.M.; Baum, B.J. Absence of tight junction formation in an allogeneic graft cell line used for developing an engineered artificial salivary gland. Tissue Eng. 2002, 8, 871–878. [Google Scholar] [CrossRef]
- Warner, J.D.; Peters, C.G.; Saunders, R.; Won, J.H.; Betzenhauser, M.J.; Gunning, W.T.; Yule, D.I.; Giovannucci, D.R. Visualizing form and function in organotypic slices of the adult mouse parotid gland. Am. J. Physiol. Gastrointest. Liver. Physiol. 2008, 295, G629–G640. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, T.; Hayashi, Y.; Nagamine, S.; Yoshida, H.; Yura, Y.; Sato, M. Generation of cells with phenotypes of both intercalated duct-type and myoepithelial cells in human parotid gland adenocarcinoma clonal cells grown in athymic nude mice. Virchows Arch. B Cell Pathol. 1986, 51, 187–195. [Google Scholar] [CrossRef]
- Shirasuna, K.; Sato, M.; Miyazaki, T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer 1981, 48, 745–752. [Google Scholar] [CrossRef]
- Pontes-Quero, S.; Heredia, L.; Casquero-García, V.; Fernández-Chacón, M.; Luo, W.; Hermoso, A.; Bansal, M.; Garcia-Gonzalez, I.; Sanchez-Muñoz, M.S.; Perea, J.R.; et al. Dual ifgMosaic: A Versatile Method for Multispectral and Combinatorial Mosaic Gene-Function Analysis. Cell 2017, 170, 800.e18–814.e18. [Google Scholar] [CrossRef]
- Snippert, H.J.; van der Flier, L.G.; Sato, T.; van Es, J.H.; van den Born, M.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; van Rheenen, J.; Simons, B.D.; et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010, 143, 134–144. [Google Scholar] [CrossRef]
- Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999, 21, 70–71. [Google Scholar] [CrossRef]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef]
- Mosier, A.P.; Peters, S.B.; Larsen, M.; Cady, N.C. Microfluidic platform for the elastic characterization of mouse submandibular glands by atomic force microscopy. Biosensors 2014, 4, 18–27. [Google Scholar] [CrossRef]
- Kong, J.; Luo, Y.; Jin, D.; An, F.; Zhang, W.; Liu, L.; Li, J.; Fang, S.; Li, X.; Yang, X.; et al. A novel microfluidic model can mimic organ-specific metastasis of circulating tumor cells. Oncotarget 2016, 7, 78421–78432. [Google Scholar] [CrossRef]




| Type | Origin | Most Common Location | Metastatic | 5-Year Survival Rate | 15-Year Survival Rate | Molecular Targets and Pathway Activation |
|---|---|---|---|---|---|---|
| Mucoepidermoid Carcinoma | Excretory stem cells | PG | Yes, regional lymphnodes | 22–86% | -- | Notch Hes1 CREB EGFR P53 Krt5 |
| Adenoid Cystic Carcinoma | Intercalated stem cells | minor SG | Yes, lungs | 89% | 40% | Notch DNAmet TGF-β c-Kit Myb p63 |
| Acinic Cell Carcinoma | Intercalated stem cells | PG | 76% | 55% | ||
| Polymorphous Adenocarcinoma | Intercalated ductal cells | Minor SG | Rarely Perineural lymphnodes | -- | -- | p63 |
| Squamous Cell Carcinoma | Excretory stem cells | PG, SMG | Neck region | -- | -- | |
| Non-Hodgkin lymphoma | Infiltrating immune cells | PG | -- | -- | ||
| Pleomorphic adenoma | Intercalated stem cells | PG | no | -- | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. https://doi.org/10.3390/cells8090976
Porcheri C, Mitsiadis TA. Physiology, Pathology and Regeneration of Salivary Glands. Cells. 2019; 8(9):976. https://doi.org/10.3390/cells8090976
Chicago/Turabian StylePorcheri, Cristina, and Thimios A. Mitsiadis. 2019. "Physiology, Pathology and Regeneration of Salivary Glands" Cells 8, no. 9: 976. https://doi.org/10.3390/cells8090976
APA StylePorcheri, C., & Mitsiadis, T. A. (2019). Physiology, Pathology and Regeneration of Salivary Glands. Cells, 8(9), 976. https://doi.org/10.3390/cells8090976