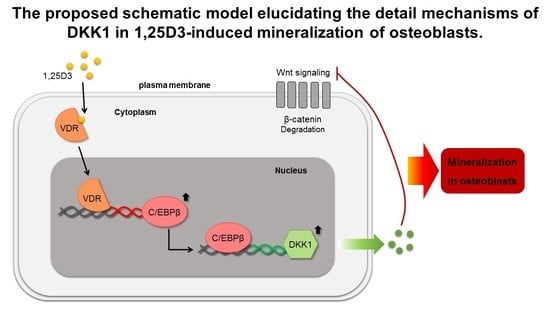
DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Isolation of Primary Osteoprogenitors, Osteoblasts, Osteoblast Differentiation
2.3. Microarray Data
2.4. In Vitro Cell Line
2.5. Constructs, Transfection, and Reagents
2.6. Immunoblotting (IB) and mRNA Analysis
2.7. Immunofluorescence (IF)
2.8. Luciferase Assay
2.9. Trichloroacetic Acid (TCA) Precipitation
2.10. Chromatin Immunoprecipitation (ChIP) Assay
2.11. Statistical Analysis
3. Results
3.1. 1,25D3 Transiently Increases Expression of DKK1 and C/EBPβ during Osteoblast Differentiation
3.2. 1,25D3 Induces Expression of DKK1 in Osteoblasts through C/EBPβ
3.3. C/EBPβ Regulates 1,25D3-Induced DKK1 Expression
3.4. 1,25D3 Stimulates Secretion of DKK1 Protein in Osteoblasts.
3.5. DKK1 Blockade Inhibits Mineralization of Osteoblast Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Willett, W.C.; Wong, J.B.; Giovannucci, E.; Dietrich, T.; Dawson-Hughes, B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA 2005, 293, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Kim, S.S.; Suh, W.Y.; Kim, J.S.; Jung, J.G.; Yoon, S.J.; Seo, Y.R.; Yang, H.J. The Effect of Education and Vitamin D Supplementation on the Achievement of Optimal Vitamin D Level in Korean Postmenopausal Women. J. Bone Metab. 2019, 26, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E.; Lutz, L.J.; Shcherbina, A.; Ricke, D.O.; Petrovick, M.; Cropper, T.L.; Cable, S.J.; McClung, J.P. Association Between Single Gene Polymorphisms and Bone Biomarkers and Response to Calcium and Vitamin D Supplementation in Young Adults Undergoing Military Training. J. Bone Miner. Res. 2017, 32, 498–507. [Google Scholar] [CrossRef]
- Bjork, A.; Mellstrom, D.; Ohlsson, C.; Karlsson, M.; Mallmin, H.; Johansson, G.; Ljunggren, O.; Kindmark, A. Haplotypes in the CYP2R1 gene are associated with levels of 25(OH)D and bone mineral density, but not with other markers of bone metabolism (MrOS Sweden). PLoS ONE 2018, 13, e0209268. [Google Scholar] [CrossRef] [Green Version]
- Arabi, A.; Khoueiry-Zgheib, N.; Awada, Z.; Mahfouz, R.; Al-Shaar, L.; Hoteit, M.; Rahme, M.; Baddoura, R.; Halabi, G.; Singh, R.; et al. CYP2R1 polymorphisms are important modulators of circulating 25-hydroxyvitamin D levels in elderly females with vitamin insufficiency, but not of the response to vitamin D supplementation. Osteoporos. Int. 2017, 28, 279–290. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Walton, R.T.; Griffiths, C.J.; Martineau, A.R. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J. Steroid. Biochem. Mol. Biol. 2016, 164, 18–29. [Google Scholar] [CrossRef]
- Ryan, J.W.; Reinke, D.; Kogawa, M.; Turner, A.G.; Atkins, G.J.; Anderson, P.H.; Morris, H.A. Novel targets of vitamin D activity in bone: Action of the vitamin D receptor in osteoblasts, osteocytes and osteoclasts. Curr. Drug Targets 2013, 14, 1683–1688. [Google Scholar] [CrossRef]
- Gronowicz, G.; Egan, J.J.; Rodan, G.A. The effect of 1,25-dihydroxyvitamin D3 on the cytoskeleton of rat calvaria and rat osteosarcoma (ROS 17/2.8) osteoblastic cells. J. Bone Miner. Res. 1986, 1, 441–455. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Lee, C.H.; Pike, J.W. Genomic determinants of gene regulation by 1,25-dihydroxyvitamin D3 during osteoblast-lineage cell differentiation. J. Biol. Chem. 2014, 289, 19539–19554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamichi, Y.; Udagawa, N.; Horibe, K.; Mizoguchi, T.; Yamamoto, Y.; Nakamura, T.; Hosoya, A.; Kato, S.; Suda, T.; Takahashi, N. VDR in Osteoblast-Lineage Cells Primarily Mediates Vitamin D Treatment-Induced Increase in Bone Mass by Suppressing Bone Resorption. J. Bone Miner. Res. 2017, 32, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Benkusky, N.A.; Lee, S.M.; St John, H.; Carlson, A.; Onal, M.; Shamsuzzaman, S. Genomic Determinants of Vitamin D-Regulated Gene Expression. Vitam. Horm. 2016, 100, 21–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Peppel, J.; van Leeuwen, J.P. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 5, 137. [Google Scholar] [CrossRef] [Green Version]
- Marchwicka, A.; Marcinkowska, E. Regulation of Expression of CEBP Genes by Variably Expressed Vitamin D Receptor and Retinoic Acid Receptor alpha in Human Acute Myeloid Leukemia Cell Lines. Int. J. Mol. Sci. 2018, 19, 1918. [Google Scholar] [CrossRef] [Green Version]
- Arensman, M.D.; Nguyen, P.; Kershaw, K.M.; Lay, A.R.; Ostertag-Hill, C.A.; Sherman, M.H.; Downes, M.; Liddle, C.; Evans, R.M.; Dawson, D.W. Calcipotriol Targets LRP6 to Inhibit Wnt Signaling in Pancreatic Cancer. Mol. Cancer Res. 2015, 13, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Luo, W.; Bunch, B.L.; Pratt, R.N.; Trump, D.L.; Johnson, C.S. 1,25D3 differentially suppresses bladder cancer cell migration and invasion through the induction of miR-101-3p. Oncotarget 2017, 8, 60080–60093. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, O.; Pena, C.; Garcia, J.M.; Larriba, M.J.; Ordonez-Moran, P.; Navarro, D.; Barbachano, A.; Lopez de Silanes, I.; Ballestar, E.; Fraga, M.F.; et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007, 28, 1877–1884. [Google Scholar] [CrossRef]
- Yao, G.Q.; Wu, J.J.; Troiano, N.; Insogna, K. Targeted overexpression of Dkk1 in osteoblasts reduces bone mass but does not impair the anabolic response to intermittent PTH treatment in mice. J. Bone Miner. Metab. 2011, 29, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Morvan, F.; Boulukos, K.; Clement-Lacroix, P.; Roman Roman, S.; Suc-Royer, I.; Vayssiere, B.; Ammann, P.; Martin, P.; Pinho, S.; Pognonec, P.; et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J. Bone Miner. Res. 2006, 21, 934–945. [Google Scholar] [CrossRef]
- Jin, H.; Wang, B.; Li, J.; Xie, W.; Mao, Q.; Li, S.; Dong, F.; Sun, Y.; Ke, H.Z.; Babij, P.; et al. Anti-DKK1 antibody promotes bone fracture healing through activation of beta-catenin signaling. Bone 2015, 71, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Preisegger, K.H.; Trajanoski, Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics 2007, 8, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, L.H.; Secreto, F.J.; Westendorf, J.J. Wnt signaling as a therapeutic target for bone diseases. Expert Opin. Ther. Targets 2009, 13, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Zhan, Z.; Li, S.; Zheng, Z.; Wang, T.; Zhang, K.; Pan, H.; Li, Z.; Zhang, N.; et al. Inflammation Intensity-Dependent Expression of Osteoinductive Wnt Proteins Is Critical for Ectopic New Bone Formation in Ankylosing Spondylitis. Arthritis Rheumatol. 2018, 70, 1056–1070. [Google Scholar] [CrossRef]
- Tominaga, H.; Maeda, S.; Hayashi, M.; Takeda, S.; Akira, S.; Komiya, S.; Nakamura, T.; Akiyama, H.; Imamura, T. CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol. Biol. Cell 2008, 19, 5373–5386. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, S.; Javed, A.; Tennant, D.K.; van Rees, M.; Montecino, M.; Stein, G.S.; Stein, J.L.; Lian, J.B. CCAAT/enhancer-binding proteins (C/EBP) beta and delta activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J. Biol. Chem. 2002, 277, 1316–1323. [Google Scholar] [CrossRef] [Green Version]
- Hata, K.; Nishimura, R.; Ueda, M.; Ikeda, F.; Matsubara, T.; Ichida, F.; Hisada, K.; Nokubi, T.; Yamaguchi, A.; Yoneda, T. A CCAAT/enhancer binding protein beta isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol. Cell. Biol. 2005, 25, 1971–1979. [Google Scholar] [CrossRef] [Green Version]
- Hirata, M.; Kugimiya, F.; Fukai, A.; Ohba, S.; Kawamura, N.; Ogasawara, T.; Kawasaki, Y.; Saito, T.; Yano, F.; Ikeda, T.; et al. C/EBPbeta Promotes transition from proliferation to hypertrophic differentiation of chondrocytes through transactivation of p57. PLoS ONE 2009, 4, e4543. [Google Scholar] [CrossRef] [Green Version]
- Shirakawa, K.; Maeda, S.; Gotoh, T.; Hayashi, M.; Shinomiya, K.; Ehata, S.; Nishimura, R.; Mori, M.; Onozaki, K.; Hayashi, H.; et al. CCAAT/enhancer-binding protein homologous protein (CHOP) regulates osteoblast differentiation. Mol. Cell. Biol. 2006, 26, 6105–6116. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.; Lee, Y.Y.; Han, J.; Lee, Y.L.; Yoon, S.; Lee, J.; Oh, Y.; Han, J.S.; Sung, I.H.; Park, Y.S.; et al. CCAAT/enhancer-binding protein beta (C/EBPbeta) is an important mediator of 1,25 dihydroxyvitamin D3 (1,25D3)-induced receptor activator of nuclear factor kappa-B ligand (RANKL) expression in osteoblasts. BMB Rep. 2019, 52, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, Y.J.; Choi, Y.S.; Kang, E.H.; Shin, K.; Kim, T.K.; Song, Y.W.; Lee, Y.J. SOCS1 suppresses IL-1beta-induced C/EBPbeta expression via transcriptional regulation in human chondrocytes. Exp. Mol. Med. 2016, 48, e241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Park, J.H.; Won, H.Y.; Lee, J.Y.; Kong, G. CBX7 inhibits breast tumorigenicity through DKK-1-mediated suppression of the Wnt/beta-catenin pathway. FASEB J 2015, 29, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.A.; Gundle, R.; Beresford, J.N. Isolation and culture of bone-forming cells (osteoblasts) from human bone. Methods Mol. Med. 1996, 2, 233–262. [Google Scholar] [CrossRef]
- Wrobel, E.; Leszczynska, J.; Brzoska, E. The Characteristics Of Human Bone-Derived Cells (HBDCS) during osteogenesis in vitro. Cell. Mol. Biol. Lett. 2016, 21, 26. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.; Lee, J.K.; Han, J.; Lee, B.; Kang, S.; Hwang, K.T.; Park, Y.S.; Kim, T.H. Identification and characterization of human bone-derived cells. Biochem. Biophys. Res. Commun. 2018, 495, 1257–1263. [Google Scholar] [CrossRef]
- Jo, S.; Wang, S.E.; Lee, Y.L.; Kang, S.; Lee, B.; Han, J.; Sung, I.H.; Park, Y.S.; Bae, S.C.; Kim, T.H. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res. Ther. 2018, 20, 115. [Google Scholar] [CrossRef]
- Koontz, L. TCA precipitation. Methods Enzymol. 2014, 541, 3–10. [Google Scholar] [CrossRef]
- Ormsby, R.T.; Findlay, D.M.; Kogawa, M.; Anderson, P.H.; Morris, H.A.; Atkins, G.J. Analysis of vitamin D metabolism gene expression in human bone: Evidence for autocrine control of bone remodelling. J. Steroid. Biochem. Mol. Biol. 2014, 144 Pt A, 110–113. [Google Scholar] [CrossRef]
- Takahashi, N. Mechanism of inhibitory action of eldecalcitol, an active vitamin D analog, on bone resorption in vivo. J. Steroid. Biochem. Mol. Biol. 2013, 136, 171–174. [Google Scholar] [CrossRef]
- Harada, S.; Matsumoto, T.; Ogata, E. Role of ascorbic acid in the regulation of proliferation in osteoblast-like MC3T3-E1 cells. J. Bone Miner. Res. 1991, 6, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, R.T.; Iyer, B.S.; Cui, Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J. Bone Miner. Res. 1994, 9, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sarosi, I.; Cattley, R.C.; Pretorius, J.; Asuncion, F.; Grisanti, M.; Morony, S.; Adamu, S.; Geng, Z.; Qiu, W.; et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 2006, 39, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, P.; Liu, W.; Maye, P.; Zhang, J.; Zhang, Y.; Hurley, M.; Guo, C.; Boskey, A.; Sun, L.; et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat. Genet. 2005, 37, 945–952. [Google Scholar] [CrossRef]
- D’Amico, L.; Mahajan, S.; Capietto, A.H.; Yang, Z.; Zamani, A.; Ricci, B.; Bumpass, D.B.; Meyer, M.; Su, X.; Wang-Gillam, A.; et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 2016, 213, 827–840. [Google Scholar] [CrossRef]
- Fulciniti, M.; Tassone, P.; Hideshima, T.; Vallet, S.; Nanjappa, P.; Ettenberg, S.A.; Shen, Z.; Patel, N.; Tai, Y.T.; Chauhan, D.; et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 2009, 114, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Plate, L.; Wiseman, R.L. Regulating Secretory Proteostasis through the Unfolded Protein Response: From Function to Therapy. Trends Cell Biol. 2017, 27, 722–737. [Google Scholar] [CrossRef]





| Antigen | Manufacturer | Species, Type | Catalog Number | Dilution |
|---|---|---|---|---|
| DKK1 | Santa Cruz/TX, USA | Mouse monoclonal | sc-374574 | 1:1000 |
| DKK1 | Cell Signaling/MA, USA | Rabbit monoclonal | 48367 | 1:1000 |
| C/EBPβ | Santa Cruz/TX, USA | Mouse monoclonal | sc-7962 | 1:1000 |
| VDR | Cell Signaling/MA, USA | Rabbit monoclonal | 12550 | 1:1000 |
| Active β-catenin | Cell Signaling/MA, USA | Rabbit monoclonal | 19807 | 1:1000 |
| β-catenin | Cell Signaling/MA, USA | Rabbit polyclonal | 9562 | 1:1000 |
| RUNX2 | Cell Signaling/MA, USA | Rabbit monoclonal | 12556 | 1:1000 |
| RUNX2 | Santa Cruz/TX, USA | Mouse monoclonal | sc-101145 | 1:1000 |
| Osteonectin, ON | Santa Cruz/TX, USA | Mouse monoclonal | sc-73472 | 1:1000 |
| Osteopontin, OPN | R&D System/MN, USA | Goat polyclonal | AF1433 | 1:2000 |
| Osteocalcin, OCN | Santa Cruz/TX, USA | Mouse monoclonal | sc-365797 | 1:1000 |
| Osteocalcin, OCN | Abcam/Cambridge, UK | Mouse monoclonal | ab13420 | 1:1000 |
| IgG | Millipore/MA, USA | Rabbit monoclonal | pp64 | 1:10000 |
| β-actin | Cell Signaling/MA, USA | Rabbit monoclonal | 4970 | 1:10000 |
| β-actin | Cell Signaling/MA, USA | Mouse monoclonal | 3700 | 1:10000 |
| GAPDH | Cell Signaling/MA, USA | Rabbit monoclonal | 2118 | 1:10000 |
| Alexa-488 | ThermoFisher/MA, USA | Mouse | A11001 | 1:100 |
| Cy3 | ThermoFisher/MA, USA | Rabbit | A10520 | 1:100 |
| HRP | Jackson ImmunoResearch/CA, USA | Mouse | 115-035-003 | 1:2000 |
| HRP | Jackson ImmunoResearch/CA, USA | Rabbit | 111-035-003 | 1:2000 |
| Gene | 5′-Forward-3′ | 5′-Reverse-3′ |
|---|---|---|
| DKK1 (mRNA) | CACACCAAAGGACAAGAAGG | CAAGACAGACCTTCTCCACA |
| C/EBPβ (mRNA) | CGACGAGTACAAGATCCGGC | TGCTTGAACAAGTTCCGCAG |
| VDR (mRNA) | TGGAGACTTTGACCGGAACG | GGGCAGGTGAATAGTGCCTT |
| ALP (mRNA) | ACGAGCTGAACAGGAACAACGT | CACCAGCAAGAAGAAGCCTTTG |
| RUNX2 (mRNA) | GTGGCCTTCAAGGTGGTAG | ACTCTTGCCTCGTCCACTC |
| COL1A1 (mRNA) | AGTGGTTTGGATGGTGCCAA | GCACCATCATTTCCACGAGC |
| ON (mRNA) | GGATGAGAACAACACCCCCA | TTTGCAAGGCCCGATGTAGT |
| OPN (mRNA) | AGCAGCTTTACAACAAATACCCAG | TTACTTGGAAGGGTCTGTGGG |
| OCN (mRNA) | AGCCACCGAGACACCATGAGA | CTCCTGAAAGCCGATGTGGTC |
| DKK2 (mRNA) | GAGGTATTGCCACAGTCCCC | GATGCCATTATTGCAGCGGG |
| DKK3 (mRNA) | TATGTGTGCAAGCCGACCTT | CTCCTCCATGAAGCTGCCAA |
| DKK4 (mRNA) | CTGTGCTACATGTCGTGGGT | TCCTTCTGCATGTGTGCCAT |
| GAPDH (mRNA) | CAAGATCATCAGCAATGCC | CTGTGGTCATGAGTCCTTCC |
| DKK1 promoter 1 (ChIP) | TTTGTATTCACTGTGCCCCTCC | CCTAGAGCCCTGGCATTGG |
| DKK1 promoter 2 (ChIP) | TCCACACACCAATTTCAATGACG | GGGACCACGCAATACCCTTT |
| DKK1 promoter 3 (ChIP) | TCTAAACGCCAGTCTCTCGC | CGGCTTTGAGGTCCTTCAGT |
| DKK1 promoter 4 (ChIP) | ACCTCAAAGCCGGGGATCTA | TTGCCCCTCTCCTTTATGCC |
| siRNA | 5′-Sense-3′ | 5′-Antisense-3′ |
| siControl (siCON) | CCUCGUGCCGUUCCAUCAGGUAGUU | CUACCUGAUGGAACGGCACGAGGUU |
| siC/EBPβ | ACAACAUCGCCGUGCGCAAUU | UUGCGCACGGCGAUGUUGUUU |
| siVDR | GGAGUUCAUUCUGACAGAUUU | AUCUGUCAGAAUGAACUCCUU |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Yoon, S.; Lee, S.Y.; Kim, S.Y.; Park, H.; Han, J.; Choi, S.H.; Han, J.-S.; Yang, J.-H.; Kim, T.-H. DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts. Cells 2020, 9, 236. https://doi.org/10.3390/cells9010236
Jo S, Yoon S, Lee SY, Kim SY, Park H, Han J, Choi SH, Han J-S, Yang J-H, Kim T-H. DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts. Cells. 2020; 9(1):236. https://doi.org/10.3390/cells9010236
Chicago/Turabian StyleJo, Sungsin, Subin Yoon, So Young Lee, So Yeon Kim, Hyosun Park, Jinil Han, Sung Hoon Choi, Joong-Soo Han, Jae-Hyuk Yang, and Tae-Hwan Kim. 2020. "DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts" Cells 9, no. 1: 236. https://doi.org/10.3390/cells9010236
APA StyleJo, S., Yoon, S., Lee, S. Y., Kim, S. Y., Park, H., Han, J., Choi, S. H., Han, J. -S., Yang, J. -H., & Kim, T. -H. (2020). DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts. Cells, 9(1), 236. https://doi.org/10.3390/cells9010236