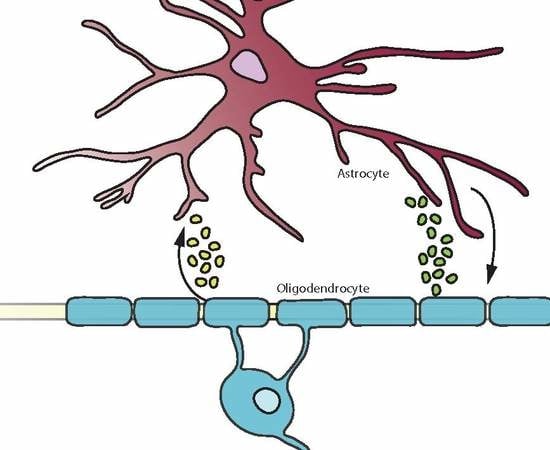
Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System
Abstract
:1. Introduction
2. Astrocyte and Oligodendrocyte Cross-Talk during Brain Development
3. Astrocytic Communication with Oligodendrocytes
3.1. Blood-Brain Barrier Interactions
3.2. Gap Junctions Connect Astrocytes and Oligodendrocytes
4. Astrocytes and Oligodendrocytes Play Active Roles in Immune Responses
5. Astrocyte—Oligodendrocyte Interplay in Disease
5.1. Reactive Gliosis and Glial Scar Formation
5.2. Astrocytes in Neuroinflammation
5.3. Excitotoxicity
6. Astrocyte Control of Remyelination and the Extracellular Matrix
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiray, H.; Lindsay, S.L.; Hosseinzadeh, S.; Barnett, S.C. The multifaceted role of astrocytes in regulating myelination. Exp. Neurol. 2016, 283, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, L.; Chu, Y.; Namaka, M.; Deng, B.; Kong, J.; Bi, X. Astrocytes in Oligodendrocyte Lineage Development and White Matter Pathology. Front. Cell Neurosci. 2016, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seth, P.; Koul, N. Astrocyte, the star avatar: Redefined. J. Biosci. 2008, 33, 405–421. [Google Scholar] [CrossRef]
- Orthmann-Murphy, J.L.; Abrams, C.K.; Scherer, S.S. Gap junctions couple astrocytes and oligodendrocytes. J. Mol. Neurosci. 2008, 35, 101–116. [Google Scholar] [CrossRef] [Green Version]
- McTigue, D.M.; Tripathi, R.B. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008, 107, 1–19. [Google Scholar] [CrossRef]
- Patel, J.; Balabanov, R. Molecular mechanisms of oligodendrocyte injury in multiple sclerosis and experimental autoimmune encephalomyelitis. Int. J. Mol. Sci. 2012, 13, 10647–10659. [Google Scholar] [CrossRef]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef]
- van der Knaap, M.S.; Bugiani, M. Leukodystrophies: A proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol. 2017, 134, 351–382. [Google Scholar] [CrossRef] [Green Version]
- Bouchat, J.; Couturier, B.; Marneffe, C.; Gankam-Kengne, F.; Balau, B.; De Swert, K.; Brion, J.-P.; Poncelet, L.; Gilloteaux, J.; Nicaise, C. Regional oligodendrocytopathy and astrocytopathy precede myelin loss and blood–brain barrier disruption in a murine model of osmotic demyelination syndrome. Glia 2018, 66, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Gankam Kengne, F.; Nicaise, C.; Soupart, A.; Boom, A.; Schiettecatte, J.; Pochet, R.; Brion, J.P.; Decaux, G. Astrocytes are an early target in osmotic demyelination syndrome. J. Am. Soc. Nephrol. 2011, 22, 1834–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gankam-Kengne, F.; Couturier, B.S.; Soupart, A.; Brion, J.P.; Decaux, G. Osmotic Stress-Induced Defective Glial Proteostasis Contributes to Brain Demyelination after Hyponatremia Treatment. J. Am. Soc. Nephrol. 2017, 28, 1802–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulter, D.A.; Eid, T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 2012, 60, 1215–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, G.; Schilling, K.; Steinhauser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Faideau, M.; Kim, J.; Cormier, K.; Gilmore, R.; Welch, M.; Auregan, G.; Dufour, N.; Guillermier, M.; Brouillet, E.; Hantraye, P.; et al. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: A correlation with Huntington’s disease subjects. Hum. Mol. Genet. 2010, 19, 3053–3067. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Lace, G.; Forster, G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B.; Function, M.R.C.C.; et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging 2010, 31, 578–590. [Google Scholar] [CrossRef]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. New Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [CrossRef]
- Moore, C.S.; Abdullah, S.L.; Brown, A.; Arulpragasam, A.; Crocker, S.J. How factors secreted from astrocytes impact myelin repair. Neurosci. Res. 2011, 89, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ponath, G.; Park, C.; Pitt, D. The Role of Astrocytes in Multiple Sclerosis. Front. Immunol. 2018, 9, 217. [Google Scholar] [CrossRef]
- Torkildsen, O.; Myhr, K.M.; Bo, L. Disease-modifying treatments for multiple sclerosis - a review of approved medications. Eur. J. Neurol. 2016, 23, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Hostenbach, S.; Cambron, M.; D’Haeseleer, M.; Kooijman, R.; De Keyser, J. Astrocyte loss and astrogliosis in neuroinflammatory disorders. Neurosci. Lett. 2014, 565, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Lucchinetti, C.F. Pathology of demyelinating diseases. Annu. Rev. Pathol. 2012, 7, 185–217. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, A.; Mori, M.; Arai, K.; Sato, Y.; Hayakawa, S.; Masuda, S.; Taniguchi, J.; Kuwabara, S. Cytokine and chemokine profiles in neuromyelitis optica: Significance of interleukin-6. Mult. Scler. 2010, 16, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, B.K.; Rosemberg, S.; Arita, F.N.; da Rocha, A.J.; Forte, W.C.N.; Tilbery, C.P. Multiphasic disseminated encephalomyelitis associated with herpes virus infection in a patient with TLR3 deficiency. Mult. Scler. Relat. Disord. 2019, 36, 101379. [Google Scholar] [CrossRef]
- Robinson, C.A.; Adiele, R.C.; Tham, M.; Lucchinetti, C.F.; Popescu, B.F. Early and widespread injury of astrocytes in the absence of demyelination in acute haemorrhagic leukoencephalitis. Acta Neuropathol. Commun. 2014, 2, 52. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Gold, R.; Adams, O.; Lassmann, H. Progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome (IRIS). Acta Neuropathol. 2015, 130, 751–764. [Google Scholar] [CrossRef]
- Gheuens, S.; Wuthrich, C.; Koralnik, I.J. Progressive multifocal leukoencephalopathy: Why gray and white matter. Annu. Rev. Pathol. 2013, 8, 189–215. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Atlas, S.W.; Green, A.J.; Bollen, A.W.; Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. New Engl. J. Med. 2005, 353, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, E.; Szpak, G.M.; Lechowicz, W.; Pasennik, E.; Sobczyk, W. Ultrastructural changes in neuronal and glial cells in subacute sclerosing panencephalitis: Correlation with disease duration. Folia Neuropathol. 2001, 39, 193–202. [Google Scholar] [PubMed]
- Mesquita, R.; Castanos-Velez, E.; Biberfeld, P.; Troian, R.M.; de Siqueira, M.M. Measles virus antigen in macrophage/microglial cells and astrocytes of subacute sclerosing panencephalitis. Apmis Acta Pathol. Microbiol. Et Immunol. Scand. 1998, 106, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, T.; Morganti-Kossmann, M.C.; Orenstein, J.M.; Britt, W.J.; Wahl, S.M.; Smith, P.D. Cytomegalovirus production by infected astrocytes correlates with transforming growth factor-beta release. J. Infect. Dis. 2003, 187, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, L.; Wang, B.; Qian, D.M.; Song, X.X.; Hu, M. HCMV induces dysregulation of glutamate uptake and transporter expression in human fetal astrocytes. Neurochem. Res. 2014, 39, 2407–2418. [Google Scholar] [CrossRef]
- Banjara, M.; Ghosh, C.; Dadas, A.; Mazzone, P.; Janigro, D. Detection of brain-directed autoantibodies in the serum of non-small cell lung cancer patients. PLoS ONE 2017, 12, e0181409. [Google Scholar] [CrossRef]
- Fang, B.; McKeon, A.; Hinson, S.R.; Kryzer, T.J.; Pittock, S.J.; Aksamit, A.J.; Lennon, V.A. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol. 2016, 73, 1297–1307. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Binswanger’s disease: Biomarkers in the inflammatory form of vascular cognitive impairment and dementia. J. Neurochem. 2018, 144, 634–643. [Google Scholar] [CrossRef]
- Pregnolato, S.; Chakkarapani, E.; Isles, A.R.; Luyt, K. Glutamate Transport and Preterm Brain Injury. Front. Physiol. 2019, 10, 417. [Google Scholar] [CrossRef] [Green Version]
- Revuelta, M.; Elicegui, A.; Moreno-Cugnon, L.; Buhrer, C.; Matheu, A.; Schmitz, T. Ischemic stroke in neonatal and adult astrocytes. Mech. Ageing Dev. 2019, 183, 111147. [Google Scholar] [CrossRef]
- Wang, H.F.; Liu, X.K.; Li, R.; Zhang, P.; Chu, Z.; Wang, C.L.; Liu, H.R.; Qi, J.; Lv, G.Y.; Wang, G.Y.; et al. Effect of glial cells on remyelination after spinal cord injury. Neural. Regen Res. 2017, 12, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Frati, G.; Luciani, M.; Meneghini, V.; De Cicco, S.; Stahlman, M.; Blomqvist, M.; Grossi, S.; Filocamo, M.; Morena, F.; Menegon, A.; et al. Human iPSC-based models highlight defective glial and neuronal differentiation from neural progenitor cells in metachromatic leukodystrophy. Cell Death Dis. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruska, N.; Schonfeld, P.; Pujol, A.; Reiser, G. Astrocytes and mitochondria from adrenoleukodystrophy protein (ABCD1)-deficient mice reveal that the adrenoleukodystrophy-associated very long-chain fatty acids target several cellular energy-dependent functions. Biochim. Et Biophys. Acta 2015, 1852, 925–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gortz, A.L.; Peferoen, L.A.N.; Gerritsen, W.H.; van Noort, J.M.; Bugiani, M.; Amor, S. Heat shock protein expression in cerebral X-linked adrenoleukodystrophy reveals astrocyte stress prior to myelin loss. Neuropathol. Appl. Neurobiol. 2018, 44, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Weidenheim, K.M.; Dickson, D.W.; Rapin, I. Neuropathology of Cockayne syndrome: Evidence for impaired development, premature aging, and neurodegeneration. Mech. Ageing Dev. 2009, 130, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, J.; Inoue, K.; Tomita, M.; Kurihara, A.; Morita, F.; Ikehira, H.; Tanada, S.; Yoshitome, E.; Kohno, Y. Brain N-acetylaspartate is elevated in Pelizaeus-Merzbacher disease with PLP1 duplication. Neurology 2002, 58, 237–241. [Google Scholar] [CrossRef]
- Adachi, M.; Schneck, L.; Cara, J.; Volk, B.W. Spongy degeneration of the central nervous system (van Bogaert and Bertrand type; Canavan’s disease). A review. Hum. Pathol. 1973, 4, 331–347. [Google Scholar] [CrossRef]
- Baslow, M.H.; Guilfoyle, D.N. Are astrocytes the missing link between lack of brain aspartoacylase activity and the spongiform leukodystrophy in Canavan disease? Neurochem. Res. 2009, 34, 1523–1534. [Google Scholar] [CrossRef]
- Borrett, D.; Becker, L.E. Alexander’s disease. A disease of astrocytes. Brain A J. Neurol. 1985, 367–385. [Google Scholar] [CrossRef]
- Bugiani, M.; Vuong, C.; Breur, M.; van der Knaap, M.S. Vanishing white matter: A leukodystrophy due to astrocytic dysfunction. Brain Pathol. (Zur. Switz.) 2018, 28, 408–421. [Google Scholar] [CrossRef]
- Hase, Y.; Chen, A.; Bates, L.L.; Craggs, L.J.L.; Yamamoto, Y.; Gemmell, E.; Oakley, A.E.; Korolchuk, V.I.; Kalaria, R.N. Severe white matter astrocytopathy in CADASIL. Brain Pathol. (Zur. Switz.) 2018, 28, 832–843. [Google Scholar] [CrossRef]
- Orthmann-Murphy, J.L.; Enriquez, A.D.; Abrams, C.K.; Scherer, S.S. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol. Cell Neurosci. 2007, 34, 629–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Knaap, M.S.; Valk, J. Magnetic Resonance of Myelination and Myelin Disorders; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Sloan, S.A.; Barres, B.A. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr. Opin. Neurobiol. 2014, 27, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, M.; Davies, J.E.; Mayer-Proschel, M.; Proschel, C.; Davies, S.J. Precursor cell biology and the development of astrocyte transplantation therapies: Lessons from spinal cord injury. Neurotherapeutics 2011, 8, 677–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molofsky, A.V.; Krencik, R.; Ullian, E.M.; Tsai, H.H.; Deneen, B.; Richardson, W.D.; Barres, B.A.; Rowitch, D.H. Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012, 26, 891–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, F.D.; Gauthier, A.S. Timing is everything: Making neurons versus glia in the developing cortex. Neuron 2007, 54, 357–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Higashimori, H.; Morel, L. Developmental maturation of astrocytes and pathogenesis of neurodevelopmental disorders. J. Neurodev. Disord. 2013, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Naruse, M.; Ishizaki, Y.; Ikenaka, K.; Tanaka, A.; Hitoshi, S. Origin of oligodendrocytes in mammalian forebrains: A revised perspective. J. Physiol. Sci. 2017, 67, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Rowitch, D.H.; Kriegstein, A.R. Developmental genetics of vertebrate glial-cell specification. Nature 2010, 468, 214–222. [Google Scholar] [CrossRef]
- Durand, B.; Raff, M. A cell-intrinsic timer that operates during oligodendrocyte development. Bioessays 2000, 22, 64–71. [Google Scholar] [CrossRef]
- Ortega, M.C.; Cases, O.; Merchan, P.; Kozyraki, R.; Clemente, D.; de Castro, F. Megalin mediates the influence of sonic hedgehog on oligodendrocyte precursor cell migration and proliferation during development. Glia 2012, 60, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Clemente, D.; Ortega, M.C.; Melero-Jerez, C.; de Castro, F. The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Front. Cell Neurosci. 2013, 7, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- See, J.; Zhang, X.; Eraydin, N.; Mun, S.B.; Mamontov, P.; Golden, J.A.; Grinspan, J.B. Oligodendrocyte maturation is inhibited by bone morphogenetic protein. Mol. Cell Neurosci. 2004, 26, 481–492. [Google Scholar] [CrossRef]
- Lanciotti, A.; Brignone, M.S.; Bertini, E.; Petrucci, T.C.; Aloisi, F.; Ambrosini, E. Astrocytes: Emerging Stars in Leukodystrophy Pathogenesis. Transl. Neurosci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Lundgaard, I.; Osorio, M.J.; Kress, B.T.; Sanggaard, S.; Nedergaard, M. White matter astrocytes in health and disease. Neuroscience 2014, 276, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Camargo, N.; Goudriaan, A.; van Deijk, A.F.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, K.A.; Dijkhuizen, R.M.; Mansvelder, H.D.; et al. Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef]
- Itoh, N.; Itoh, Y.; Tassoni, A.; Ren, E.; Kaito, M.; Ohno, A.; Ao, Y.; Farkhondeh, V.; Johnsonbaugh, H.; Burda, J.; et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proc. Natl. Acad. Sci. USA 2018, 115, E302–E309. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.P.; Tang, Y.; Zhou, S.; Toh, B.H.; McLean, C.; Li, H. Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol. Cell Neurosci. 2010, 43, 33–42. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci.. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Stephenson, E.; Nathoo, N.; Mahjoub, Y.; Dunn, J.F.; Yong, V.W. Iron in multiple sclerosis: Roles in neurodegeneration and repair. Nat. Rev. Neurol. 2014, 10, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Morath, D.J.; Mayer-Proschel, M. Iron deficiency during embryogenesis and consequences for oligodendrocyte generation in vivo. Dev. Neurosci. 2002, 24, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Sci. (New Yorkn.Y.) 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Dohgu, S.; Takata, F.; Matsumoto, J.; Watanabe, T.; Iwao, T.; Yamauchi, A.; Kataoka, Y. Oligodendrocytes upregulate blood-brain barrier function through mechanisms other than the PDGF-BB/PDGFRalpha pathway in the barrier-tightening effect of oligodendrocyte progenitor cells. Neurosci. Lett. 2020, 715, 134594. [Google Scholar] [CrossRef]
- Niu, J.; Tsai, H.H.; Hoi, K.K.; Huang, N.; Yu, G.; Kim, K.; Baranzini, S.E.; Xiao, L.; Chan, J.R.; Fancy, S.P.J. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat. Neurosci. 2019, 22, 709–718. [Google Scholar] [CrossRef]
- Giaume, C.; Naus, C.C. Connexins, gap junctions, and glia. Wiley Interdiscip. Rev. Membr. Transport. Signal. 2013, 2013, 133–142. [Google Scholar] [CrossRef]
- Masaki, K. Early disruption of glial communication via connexin gap junction in multiple sclerosis, Balo’s disease and neuromyelitis optica. Neuropathology 2015, 35, 469–480. [Google Scholar] [CrossRef]
- Papaneophytou, C.P.; Georgiou, E.; Karaiskos, C.; Sargiannidou, I.; Markoullis, K.; Freidin, M.M.; Abrams, C.K.; Kleopa, K.A. Regulatory role of oligodendrocyte gap junctions in inflammatory demyelination. Glia 2018, 66, 2589–2603. [Google Scholar] [CrossRef]
- Boulay, A.-C.; Mazeraud, A.; Cisternino, S.; Saubaméa, B.; Mailly, P.; Jourdren, L.; Blugeon, C.; Mignon, V.; Smirnova, M.; Cavallo, A.; et al. Immune quiescence of the brain is set by astroglial connexin 43. J. Neurosci. 2015, 35, 4427–4439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbinyan, A.; Kaminski, R.; White, M.K.; Darbinian-Sarkissian, N.; Khalili, K. Polyomavirus JC infection inhibits differentiation of oligodendrocyte progenitor cells. Neurosci. Res. 2013, 91, 116–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, G.; Benge, S.; Pahar, B.; Philipp, M.T. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. J. Neuroinflammation 2012, 9, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andjelkovic, A.V.; Song, L.; Dzenko, K.A.; Cong, H.; Pachter, J.S. Functional expression of CCR2 by human fetal astrocytes. Neurosci. Res. 2002, 70, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Ashutosh; Kou, W.; Cotter, R.; Borgmann, K.; Wu, L.; Persidsky, R.; Sakhuja, N.; Ghorpade, A. CXCL8 protects human neurons from amyloid-beta-induced neurotoxicity: Relevance to Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2011, 412, 565–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, M.P.; Frausto, R.; Rose-John, S.; Campbell, I.L. Analysis of IL-6/gp130 family receptor expression reveals that in contrast to astroglia, microglia lack the oncostatin M receptor and functional responses to oncostatin M. Glia 2015, 63, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Omari, K.M.; John, G.; Lango, R.; Raine, C.S. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia 2006, 53, 24–31. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [Green Version]
- Moynagh, P.N. The interleukin-1 signalling pathway in astrocytes: A key contributor to inflammation in the brain. J. Anat 2005, 207, 265–269. [Google Scholar] [CrossRef]
- Moyon, S.; Dubessy, A.L.; Aigrot, M.S.; Trotter, M.; Huang, J.K.; Dauphinot, L.; Potier, M.C.; Kerninon, C.; Melik Parsadaniantz, S.; Franklin, R.J.; et al. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci. 2015, 35, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kim, D.; Cui, J.; Jang, H.H.; Kim, K.S.; Lee, H.J.; Kim, S.U.; Ahn, S.M. Secretome analysis of human oligodendrocytes derived from neural stem cells. PLoS ONE 2014, 9, e84292. [Google Scholar] [CrossRef] [Green Version]
- Kadi, L.; Selvaraju, R.; de Lys, P.; Proudfoot, A.E.; Wells, T.N.; Boschert, U. Differential effects of chemokines on oligodendrocyte precursor proliferation and myelin formation in vitro. J. Neuroimmunol. 2006, 174, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Cao, D.L.; Zhang, X.; Ji, R.R.; Gao, Y.J. Chemokine contribution to neuropathic pain: Respective induction of CXCL1 and CXCR2 in spinal cord astrocytes and neurons. Pain 2013, 154, 2185–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulay, A.-C.; Cisternino, S.; Cohen-Salmon, M. Immunoregulation at the gliovascular unit in the healthy brain: A focus on Connexin 43. Brain Behaviour Immunol. 2016, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boulay, A.-C.; Gilbert, A.; Oliveira Moreira, V.; Blugeon, C.; Perrin, S.; Pouch, J.; Le Crom, S.; Ducos, B.; Cohen-Salmon, M. Connexin 43 Controls the Astrocyte Immunoregulatory Phenotype. Brain Sci. 2018, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Allaman, I.; Belanger, M.; Magistretti, P.J. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, K.H.; Park, H.S.; Kim, S.K.; Koh, C.M.; Park, J.Y. Differential expression, shedding, cytokine regulation and function of TNFR1 and TNFR2 in human fetal astrocytes. Yonsei Med. J. 2005, 46, 818–826. [Google Scholar] [CrossRef]
- Kemanetzoglou, E.; Andreadou, E. CNS Demyelination with TNF-alpha Blockers. Curr. Neurol. Neurosci. Rep. 2017, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Pegoretti, V.; Baron, W.; Laman, J.D.; Eisel, U.L.M. Selective Modulation of TNF-TNFRs Signaling: Insights for Multiple Sclerosis Treatment. Front. Immunol. 2018, 9, 925. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, D.; Fang, M.; Zhu, G.; Chen, C.; Zeng, H.; Lu, J.; Charanjit, K. Astrocyte-derived proinflammatory cytokines induce hypomyelination in the periventricular white matter in the hypoxic neonatal brain. PLoS ONE 2014, 9, e87420. [Google Scholar] [CrossRef] [Green Version]
- Miljkovic, D.; Timotijevic, G.; Mostarica Stojkovic, M. Astrocytes in the tempest of multiple sclerosis. FEBS Lett. 2011, 585, 3781–3788. [Google Scholar] [CrossRef] [Green Version]
- Burm, S.M.; Peferoen, L.A.; Zuiderwijk-Sick, E.A.; Haanstra, K.G.; t Hart, B.A.; van der Valk, P.; Amor, S.; Bauer, J.; Bajramovic, J.J. Expression of IL-1beta in rhesus EAE and MS lesions is mainly induced in the CNS itself. J. Neuroinflammation. 2016, 13, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesp, Z.C.; Goldstein, E.Z.; Miranda, C.J.; Kaspar, B.K.; McTigue, D.M. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J. Neurosci. 2015, 35, 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Panitch, H.S.; Hirsch, R.L.; Schindler, J.; Johnson, K.P. Treatment of multiple sclerosis with gamma interferon: Exacerbations associated with activation of the immune system. Neurology 1987, 37, 1097–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMorris, F.A.; Smith, T.M.; DeSalvo, S.; Furlanetto, R.W. Insulin-like growth factor I/somatomedin C: A potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA 1986, 83, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.; Yee, K.L.; Sumbria, R.K. Tumor necrosis factor alpha Inhibition for Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517709278. [Google Scholar] [CrossRef]
- Fischer, R.; Wajant, H.; Kontermann, R.; Pfizenmaier, K.; Maier, O. Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia 2014, 62, 272–283. [Google Scholar] [CrossRef]
- Hofman, F.M.; Hinton, D.R.; Johnson, K.; Merrill, J.E. Tumor necrosis factor identified in multiple sclerosis brain. J. Exp. Med. 1989, 170, 607–612. [Google Scholar] [CrossRef]
- Selmaj, K.; Raine, C.S.; Farooq, M.; Norton, W.T.; Brosnan, C.F. Cytokine cytotoxicity against oligodendrocytes. Apoptosis induced by lymphotoxin. J. Immunol. 1991, 147, 1522–1529. [Google Scholar]
- Selmaj, K.; Raine, C.S. Tumor necrosis factor mediates myelin damage in organotypic cultures of nervous tissue. Ann. N. Y. Acad. Sci. 1988, 540, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Agresti, C.; D’Urso, D.; Levi, G. Reversible inhibitory effects of interferon-gamma and tumour necrosis factor-alpha on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur. J. Neurosci. 1996, 8, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Hindinger, C.; Bergmann, C.C.; Hinton, D.R.; Phares, T.W.; Parra, G.I.; Hussain, S.; Savarin, C.; Atkinson, R.D.; Stohlman, S.A. IFN-gamma signaling to astrocytes protects from autoimmune mediated neurological disability. PLoS ONE 2012, 7, e42088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFerla, F.M.; Sugarman, M.C.; Lane, T.E.; Leissring, M.A. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-gamma. J. Mol. Neurosci. 2000, 15, 45–59. [Google Scholar] [CrossRef]
- Butt, A.M.; Dinsdale, J. Fibroblast growth factor 2 induces loss of adult oligodendrocytes and myelin in vivo. Exp. Neurol. 2005, 192, 125–133. [Google Scholar] [CrossRef]
- Gross, R.E.; Mehler, M.F.; Mabie, P.C.; Zang, Z.; Santschi, L.; Kessler, J.A. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 1996, 17, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Clarner, T.; Parabucki, A.; Beyer, C.; Kipp, M. Corticosteroids impair remyelination in the corpus callosum of cuprizone-treated mice. J. Neuroendocr. 2011, 23, 601–611. [Google Scholar] [CrossRef]
- Balabanov, R.; Strand, K.; Goswami, R.; McMahon, E.; Begolka, W.; Miller, S.D.; Popko, B. Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 2013–2024. [Google Scholar] [CrossRef] [Green Version]
- Semple, B.D.; Frugier, T.; Morganti-Kossmann, M.C. CCL2 modulates cytokine production in cultured mouse astrocytes. J. Neuroinflammation 2010, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S.H.; van der Meer, P.; Hesselgesser, J.; Jaffer, S.; Kolson, D.L.; Albright, A.V.; Gonzalez-Scarano, F.; Lavi, E. CXCR3 expression in human central nervous system diseases. Neuropathol. Appl. Neurobiol. 2001, 27, 127–138. [Google Scholar] [CrossRef]
- You, T.; Bi, Y.; Li, J.; Zhang, M.; Chen, X.; Zhang, K.; Li, J. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci. Rep. 2017, 7, 41779. [Google Scholar] [CrossRef] [PubMed]
- Narcisse, L.; Scemes, E.; Zhao, Y.; Lee, S.C.; Brosnan, C.F. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 2005, 49, 245–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeis, T.; Enz, L.; Schaeren-Wiemers, N. The immunomodulatory oligodendrocyte. Brain Res. 2016, 1641, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.K.; Park, S.Y.; Kim, K.H.; Park, S.R.; Lee, S.G.; Choi, B.H. GM-CSF reduces expression of chondroitin sulfate proteoglycan (CSPG) core proteins in TGF-beta-treated primary astrocytes. BMB Rep. 2014, 47, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prins, M.; Schul, E.; Geurts, J.; van der Valk, P.; Drukarch, B.; van Dam, A.M. Pathological differences between white and grey matter multiple sclerosis lesions. Ann. N. Y. Acad. Sci. 2015, 1351, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sosunov, A.; Olabarria, M.; Goldman, J.E. Alexander disease: An astrocytopathy that produces a leukodystrophy. Brain Pathol. (Zur. Switz.) 2018, 28, 388–398. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.B.; Zhuge, Q.; Zheng, W.; Shao, B.; Wang, B.; Sun, F.; Jin, K. Glial scar formation occurs in the human brain after ischemic stroke. Int. J. Med. Sci. 2014, 11, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Verkhratsky, A.; Parpura, V.; Pekna, M.; Pekny, M.; Sofroniew, M. Glia in the pathogenesis of neurodegenerative diseases. Biochem. Soc. Trans. 2014, 42, 1291–1301. [Google Scholar] [CrossRef]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Ceyzériat, K.; Ben Haim, L.; Denizot, A.; Pommier, D.; Matos, M.; Guillemaud, O.; Palomares, M.-A.; Abjean, L.; Petit, F.; Gipchtein, P.; et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwin, S.K.; Rao, V.; Moore, C.S.; Antel, J.P. Astrocytes in multiple sclerosis. Mult. Scler. 2016, 22, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, S.M.; Yan, J. NF-kappaB Pathways in the Pathogenesis of Multiple Sclerosis and the Therapeutic Implications. Front. Mol. Neurosci. 2016, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Bai, Y.; Zhu, X.; Lin, L.; Zhao, L.; Wu, X.; Buch, S.; Wang, L.; Chao, J.; Yao, H. IL-17A induces MIP-1alpha expression in primary astrocytes via Src/MAPK/PI3K/NF-kB pathways: Implications for multiple sclerosis. J. Neuroimmune Pharm. 2014, 9, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Persaud, T.; Hu, X.; Karmally, S.; Shestopalov, V.I.; Dvoriantchikova, G.; Ivanov, D.; Nathanson, L.; Barnum, S.R.; Bethea, J.R. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J. Immunol. 2009, 182, 2628–2640. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sanchez, K.; Ariza-Salamanca, D.; Mora-Munoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef] [Green Version]
- Ouali Alami, N.; Schurr, C.; Olde Heuvel, F.; Tang, L.; Li, Q.; Tasdogan, A.; Kimbara, A.; Nettekoven, M.; Ottaviani, G.; Raposo, C.; et al. NF-kappaB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018, 37, e98697. [Google Scholar] [CrossRef]
- Butt, A.M.; De La Rocha, I.C.; Rivera, A. Oligodendroglial Cells in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 325–333. [Google Scholar] [CrossRef]
- Matute, C.; Torre, I.; Perez-Cerda, F.; Perez-Samartin, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodriguez-Antiguedad, A.; Sanchez-Gomez, M.; et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef]
- Bezzi, P.; Domercq, M.; Brambilla, L.; Galli, R.; Schols, D.; De Clercq, E.; Vescovi, A.; Bagetta, G.; Kollias, G.; Meldolesi, J.; et al. CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2001, 4, 702–710. [Google Scholar] [CrossRef]
- Jeremic, A.; Jeftinija, K.; Stevanovic, J.; Glavaski, A.; Jeftinija, S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J. Neurochem. 2001, 77, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Arcuino, G.; Takano, T.; Lin, J.; Peng, W.G.; Wan, P.; Li, P.; Xu, Q.; Liu, Q.S.; Goldman, S.A.; et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 2004, 10, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Amadio, S.; Parisi, C.; Piras, E.; Fabbrizio, P.; Apolloni, S.; Montilli, C.; Luchetti, S.; Ruggieri, S.; Gasperini, C.; Laghi-Pasini, F.; et al. Modulation of P2X7 Receptor during Inflammation in Multiple Sclerosis. Front. Immunol. 2017, 8, 1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamer, E.; Fischer, W.; Engel, T. The ATP-Gated P2X7 Receptor As a Target for the Treatment of Drug-Resistant Epilepsy. Front. Neurosci. 2017, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.A.; Akirav, E.M. Remyelination in multiple sclerosis: Cellular mechanisms and novel therapeutic approaches. Neurosci. Res. 2015, 93, 687–696. [Google Scholar] [CrossRef]
- Van Strien, M.E.; Baron, W.; Bakker, E.N.; Bauer, J.; Bol, J.G.; Breve, J.J.; Binnekade, R.; Van Der Laarse, W.J.; Drukarch, B.; Van Dam, A.M. Tissue transglutaminase activity is involved in the differentiation of oligodendrocyte precursor cells into myelin-forming oligodendrocytes during CNS remyelination. Glia 2011, 59, 1622–1634. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Murtie, J.C.; Ness, J.K.; Redwine, J.M.; Enterline, J.R.; Armstrong, R.C.; Levison, S.W. Astrocytes produce CNTF during the remyelination phase of viral-induced spinal cord demyelination to stimulate FGF-2 production. Neurobiol. Dis. 2003, 13, 89–101. [Google Scholar] [CrossRef]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisen, J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Mohassel, P.; Foley, A.R.; Bonnemann, C.G. Extracellular matrix-driven congenital muscular dystrophies. Matrix Biol. 2018, 71–72, 188–204. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; Dijkstra, C.D.; de Vries, H.E. The extracellular matrix in multiple sclerosis pathology. J. Neurochem. 2007, 103, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.W.; Keough, M.B.; Haylock-Jacobs, S.; Cua, R.; Döring, A.; Sloka, S.; Stirling, D.P.; Rivest, S.; Yong, V.W. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 2012, 72, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, C.; Liu, X.B.; Pleasure, D.E.; Liu, Y.; Deng, W. Human iPSC-Derived Immature Astroglia Promote Oligodendrogenesis by Increasing TIMP-1 Secretion. Cell Rep. 2016, 15, 1303–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, N.; Maki, T.; Shindo, A.; Liang, A.C.; Maeda, M.; Egawa, N.; Itoh, K.; Lo, E.K.; Lok, J.; Ihara, M.; et al. Astrocytes Promote Oligodendrogenesis after White Matter Damage via Brain-Derived Neurotrophic Factor. J. Neurosci. 2015, 35, 14002–14008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Gorter, R.P.; de Jonge, J.C.; Nazmuddin, M.; Zhao, C.; Amor, S.; Hoekstra, D.; Baron, W. MMP7 cleaves remyelination-impairing fibronectin aggregates and its expression is reduced in chronic multiple sclerosis lesions. Glia 2018, 66, 1625–1643. [Google Scholar] [CrossRef] [PubMed]


| Disease | Pathology | Detrimental Impact on Astrocytes | Beneficial Impact on Astrocytes | References | |
|---|---|---|---|---|---|
| Inflammatory | MS | Inflammation, myelin loss, neurodegeneration, astrogliosis, astrocyte damage. | BBB damage, impaired signal transduction and glutamate clearance. Reduced OPC proliferation | Gliosis may aid remyelination and regenerate integrity of BBB, aid remyelination and provide trophic support | [5,20,21,22,23] |
| NMO | Inflammation, myelin loss in optic nerve and spinal cord. Reduction in AQP4 and GFAP. Decreased EAAT2. | Impaired water and ion homeostasis, impaired glutamate clearance | Stimulation of remyelination, trophic support | [21,24,25,26] | |
| ADEM | Widespread CNS inflammation associated with infection. | Dependent on infectious agent | Infection may trigger protective response via TLR-dependent mechanism | [27] | |
| AHL | Perivascular demyelination, inflammation, oedema, haemorrhages. Hyper-reactive astrocytes. | Swelling of protoplasmic and fibrous astrocyte end-feet, beading consistent with degeneration. | Demyelination is secondary to astrocyte injury indicating a beneficial effect of astrocytes in early disease | [28] | |
| Infectious | PML | Cytolytic JC virus induces oligodendrocytes death and focal myelin loss. Abnormal astrocytes with inclusion bodies. | Astrocytes aid the spread of JC virus to neighbouring oligodendrocytes | Unknown | [29,30,31] |
| SSPE | Viral inclusion bodies in neurons, neuronal damage and loss. Virion inclusion in some astrocytes. | Infection of (perivascular) astrocytes may aid spread of virus | Reactive gliosis in longstanding disease may be beneficial | [32,33] | |
| Congenital CMV | Encephalitis, microglial activation. | CMV infection of astrocytes induces TGF-beta known to enhance productive infection. Infection of foetal astrocytes alters uptake and metabolism of glutamate | Unknown | [34,35] | |
| Toxic-Metabolic | PNND | Depends on position and type of tumour. | Pathogenic antibodies and CD8+ T cells to astrocytic antigens expressed on tumour induces neurological damage | Unknown | [36,37] |
| Hypoxia-Ischemia | Binswanger disease | Chronic microvascular leukoencephalopathy, white matter lesions, axonal damage. | Damage to BBB leads to peri-infarct reactive astrocytes | Unknown | [38] |
| Cerebral hypoxia and ischemia in new-borns | Diffuse white matter damage, gliosis, decrease in oligodendrocytes. | Reactive astrocytes form a glia scar and secret inflammatory molecules e.g., ROS | Astrocytes produce PDGF, IGF-1, elevated levels of EAAT2 aid glutamate removal in response to hypoxia. VEGF production mobilises stem cells. BDNF reduces apoptosis. | [39,40] | |
| TBI | Diffuse axonal injury | Axonal damage, tau accumulation, secondary white matter damage, astrogliosis. | Glial scar inhibits remyelination and axonal regrowth | Glial scar prevents spread of toxic molecules | [2,41] |
| Lysosomal Storage | MLD | Accumulated sulfatides leads to demyelination, sparing of U-fibres. Eosinophilic granules in macrophages, metachromasia. | Sulfatide accumulates in astrocytes impairing differentiation | Unknown | [42] |
| Peroxisomal | X-linked ALD | Defective ABCD1 transport protein. Increased saturated VLCFA in serum. Progressive demyelination. VLCFA accumulate in glia. | Astrocyte stress prior to myelin damage due to accumulated VLCFA. Astrocytes produce ROS and have impaired oxidative ATP synthesis and decreased Ca2+ uptake capacity | Unknown | [43,44] |
| Mitochondrial | Leber’s hereditary optic neuropathy | Loss of retinal ganglion cells, optic nerve degeneration. | Unknown | Unknown | |
| DNA Repair Defects | Cockayne syndrome | Patchy myelin loss, neuronal loss, astrocytic gliosis, microglia nodules. | Multinucleated astrocytes | Unknown | [45] |
| Defects in Myelin Genes | PMD | PLP1 duplication or gene alterations, dysmyelination, failure to form myelin. | Increased astrocytic activity, astrogliosis. | Unknown | [46] |
| AA/Organic Acid Metabolism Disorders | Canavan disease | Mutations of aspartoacylase gene diffuse spongiform white matter degeneration, dysmyelination and intramyelinic oedema. Hypertrophy and hyperplasia of astrocytes. | Metabolic disturbance of mitochondria in abnormal astrocyte | Unknown | [47,48] |
| Miscellaneous | Alexander disease | Myelin damage, Rosenthal fibres, non-neoplastic astrocytes | Mutations in GFAP lead to diminished glutamate transporter, accumulation of CD44, and loss of EAAT-2. Loss of Cx43 and Cx30 | Unknown | [49] |
| VWM | Progressive demyelination, blunted dysmorphic astrocytes. | Failure to reach maturity of astrocytes. Overexpression of nestin and GFAPδ | Unknown | [50] | |
| CADASIL | Diffuse white matter lesions, subcortical infarcts. Granular osmiophilic material in small vessels | Astrocytes undergo autophagy-like cell death. Glia-vascular unit damaged, BBB disturbed | Unknown | [51] | |
| PMLD | Lack of the gap junction protein Cx47 leads to splitting and decompaction of myelin sheaths and axonal spheroids. | Gap junctions between astrocytes and oligodendrocytes are disturbed compromising oligodendrocyte survival and myelination. | Unknown | [52] |
| Detrimental | Beneficial | References | |
|---|---|---|---|
| Astrocyte Mediator | Impact on Oligodendrocytes | ||
| TNF-α | Induces demyelination and oligodendrocyte necrosis | Induces PDGF, and LIF on astrocytes which enhances OPC survival and differentiation | [3,108,109,110,111,112] |
| IL-1β | Induces oligodendrocyte apoptosis and hypomyelination | [102] | |
| IFN-γ | Reversibly reduces OPC proliferation | Limits inflammation, limits Th17 activation, limits IL-1β signalling, protects oligodendrocytes from endoplasmic reticulum stress | [106,113,114,115] |
| FGF-2 | Induces loss of myelin and myelin-producing oligodendrocytes | Induces proliferation of OPCs | [4,116] |
| BMP | BMPs induce OPC differentiation into the astrocyte lineage | [21,117] | |
| CNTF | Induces proliferation and differentiation of OPCs | [21,105] | |
| IGF-1 | Induces OPC differentiation | [21,107,118] | |
| Oligodendrocyte Mediator | Impact on Astrocytes | ||
| CCL2 | Reduces IL-6 expression in astrocytes, leading to a less inflammatory environment | [92,119,120] | |
| CXCL10 | Induces CXCR3 receptor expression | [119,121] | |
| IL-17 | Induces GFAP, IL-1β, and VEGF, reduces BBB integrity Induces astrogliosis | [89,122] | |
| IL-1β | Induces IL-1β and NF-κB, and P2X7 receptor. | [90,92,123,124] | |
| GM-CSF | Inhibits glial scar formation. Induces proliferation, and migration of astrocytes | [93,125] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. https://doi.org/10.3390/cells9030600
Nutma E, van Gent D, Amor S, Peferoen LAN. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells. 2020; 9(3):600. https://doi.org/10.3390/cells9030600
Chicago/Turabian StyleNutma, Erik, Démi van Gent, Sandra Amor, and Laura A. N. Peferoen. 2020. "Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System" Cells 9, no. 3: 600. https://doi.org/10.3390/cells9030600
APA StyleNutma, E., van Gent, D., Amor, S., & Peferoen, L. A. N. (2020). Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells, 9(3), 600. https://doi.org/10.3390/cells9030600