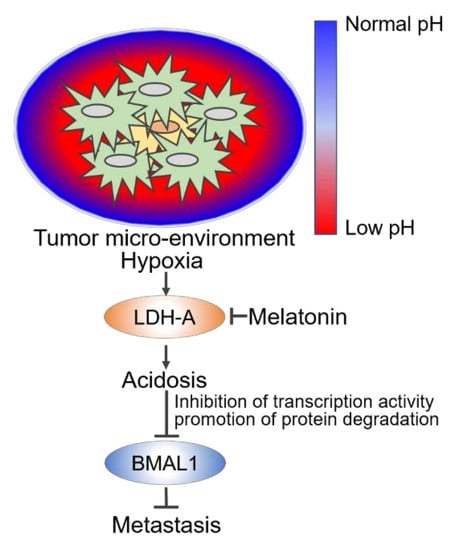
Extracellular Acidosis Promotes Metastatic Potency via Decrease of the BMAL1 Circadian Clock Gene in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Lines and Culture Conditions
2.3. Conditioned Media
2.4. pH Regulation
2.5. Cell Viability Assay
2.6. DNA and siRNA Transfection
2.7. Immunoblotting
2.8. RNA Isolation and Quantitative Real-Time PCR
2.9. Trans-Well Migration Assay
2.10. Wound-Healing Assay
2.11. Public Datasets
2.12. Statistical Analysis
3. Results
3.1. Chronic Hypoxia Reduces the BMAL1 Expression in Breast Cancer Cells
3.2. Hypoxia-Mediated Acidosis Reduces Circadian BMAL1 Expression in Breast Cancer Cells
3.3. Tumor Acidosis Reduces BMAL1 via Inhibition of Transcription Activity and Protein Stability in Breast Cancer Cells
3.4. Tumor Acidosis-Mediated Decrease of BMAL1 Promotes Metastatic Potency in Breast Cancer Cells
3.5. Melatonin Attenuates Decrease of BMAL1 by Inhibiting Hypoxia-Mediated LDH-A in Breast Cancer Cells
3.6. Decrease of BMAL1 is Clinically Related to Poor Prognoses in Breast Cancer Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar] [PubMed]
- Weigelt, B.; Peterse, J.L.; Veer, L.J.V. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Breast Cancer Facts & Figures 2017–2018; American Cancer Society: Atlanta, GA, USA, 2017. [Google Scholar]
- Khan, S.; Nabi, G.; Yao, L.; Siddique, R.; Sajjad, W.; Kumar, S.; Duan, P.; Hou, H. Health risks associated with genetic alterations in internal clock system by external factors. Int. J. Biol. Sci. 2018, 14, 791. [Google Scholar] [CrossRef] [Green Version]
- Hirano, A.; Fu, Y.H.; Ptáček, L.J. The intricate dance of post-translational modifications in the rhythm of life. Nat. Struct. Mol. Biol. 2016, 23, 1053. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453. [Google Scholar] [CrossRef]
- Fu, L.; Lee, C.C. The circadian clock: Pacemaker and tumour suppressor. Nat. Rev. Cancer 2003, 3, 350. [Google Scholar] [CrossRef]
- Foster, R.G.; Wulff, K. The rhythm of rest and excess. Nat. Rev. Neurosci. 2005, 6, 407. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, S.; Shen, J.; Guo, L.; Sun, Y.; Zhu, Y.; Ma, Z.; Zhang, X.; Hu, Y.; Xiao, W.; et al. The MiR-135b–BMAL1–YY1 loop disturbs pancreatic clockwork to promote tumourigenesis and chemoresistance. Cell Death Dis. 2018, 9, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Liu, L.; Liu, D.; Jin, S.; Yang, Y.; Tang, W.; Gong, L. Decreased circadian component Bmal1 predicts tumor progression and poor prognosis in human pancreatic ductal adenocarcinoma. Biochem. Biophys. Res. Commun. 2016, 472, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Mao, L.; Duplessis, T.; Yuan, L.; Dauchy, R.; Dauchy, E.; Blask, D.E.; Frasch, T.; Hill, S.M. Oscillation of clock and clock controlled genes induced by serum shock in human breast epithelial and breast cancer cells: Regulation by melatonin. Breast Cancer Basic Clin. Res. 2016, 6, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, S.; Esposito, J.; Corlazzoli, F.; Gregorski, A.; Sacchi, N. Entrainment of breast (cancer) epithelial cells detects distinct circadian oscillation patterns for clock and hormone receptor genes. Cell Cycle 2012, 11, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Cheng, B.; Xie, M.; Chen, Y.; Zhao, J.; Zhou, X.; Chen, L. Circadian clock gene Bmal1 inhibits tumorigenesis and increases paclitaxel sensitivity in tongue squamous cell carcinoma. Cancer Res. 2017, 77, 532–544. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.L.; Luo, H.Y.; Yang, J.; Wu, W.J.; Chen, D.L.; Huang, P.; Xu, R.H. Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin. Cancer Res. 2014, 20, 1042–1052. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Kim, E.M.; Park, J.K.; Hwang, S.G.; Moon, S.K.; Kim, W.J.; Um, H.D. Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol. Rep. 2013, 29, 2109–2113. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, T.; Aygenli, F.; Emisoglu, H.; Ozcelik, G.; Canturk, A.; Yilmaz, S.; Ozturk, N. Opposite carcinogenic effects of circadian clock gene BMAL1. Sci. Rep. 2018, 8, 16023. [Google Scholar] [CrossRef] [Green Version]
- De Assis, L.V.M.; Moraes, M.N.; Magalhães-Marques, K.K.; Kinker, G.S.; da Silveira Cruz-Machado, S.; de Lauro Castrucci, A.M. Non-metastatic cutaneous melanoma induces chronodisruption in central and peripheral circadian clocks. Int. J. Mol. Sci. 2018, 19, 1065. [Google Scholar] [CrossRef] [Green Version]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-modified cancer cell metabolism. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta 2010, 1797, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577. [Google Scholar] [CrossRef]
- Gupta, S.C.; Singh, R.; Pochampally, R.; Watabe, K.; Mo, Y.Y. Acidosis promotes invasiveness of breast cancer cells through ROS-AKT-NF-κB pathway. Oncotarget 2014, 5, 12070. [Google Scholar] [CrossRef] [Green Version]
- Damaghi, M.; Tafreshi, N.K.; Lloyd, M.C.; Sprung, R.; Estrella, V.; Wojtkowiak, J.W.; Morse, D.L.; Koomen, J.M.; Bui, M.M.; Gatenby, R.A.; et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 2015, 6, 8752. [Google Scholar] [CrossRef] [Green Version]
- Walton, Z.E.; Patel, C.H.; Brooks, R.C.; Yu, Y.; Ibrahim-Hashim, A.; Riddle, M.; Porcu, A.; Jiang, T.; Ecker, B.L.; Tameire, F.; et al. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell 2018, 174, 72–87. [Google Scholar] [CrossRef] [Green Version]
- Nagy, Á.; Lánczky, A.; Menyhárt, O.; Győrffy, B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci. Rep. 2018, 8, 9227. [Google Scholar] [CrossRef]
- Peek, C.B.; Levine, D.C.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017, 25, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Balkovetz, D.F.; Chumley, P.; Amlal, H. Downregulation of claudin-2 expression in renal epithelial cells by metabolic acidosis. Am. J. Physiol. Renal. Physiol. 2009, 297, F604–F611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissner, S.; Hagen, F.; Deiner, C.; Günzel, D.; Greco, G.; Shen, Z.; Aschenbach, J.R. Key role of short-chain fatty acids in epithelial barrier failure during ruminal acidosis. J. Dairy Sci. 2017, 100, 6662–6675. [Google Scholar] [CrossRef]
- Uthaman, S.; Huh, K.M.; Park, I.K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Kondo, A.; Yamamoto, S.; Nakaki, R.; Shimamura, T.; Hamakubo, T.; Sakai, J.; Kodama, T.; Yoshida, T.; Aburatani, H.; Osawa, T. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 2017, 18, 2228–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshetnyak, Y.K. Imaging tumor acidity: pH-low insertion peptide probe for optoacoustic tomography. Clin. Cancer Res. 2015, 21, 4502–4504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671. [Google Scholar] [CrossRef]
- Fais, S.; Venturi, G.; Gatenby, B. Microenvironmental acidosis in carcinogenesis and metastases: New strategies in prevention and therapy. Cancer Metastasis Rev. 2014, 33, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Menard, J.A.; Christianson, H.C.; Kucharzewska, P.; Bourseau-Guilmain, E.; Svensson, K.J.; Lindqvist, E.; Chandran, V.I.; Kjellén, L.; Welinder, C.; Bengzon, J.; et al. Metastasis stimulation by hypoxia and acidosis-induced extracellular lipid uptake is mediated by proteoglycan-dependent endocytosis. Cancer Res. 2016, 76, 4828–4840. [Google Scholar] [CrossRef] [Green Version]
- Kelly, N.J.; Varga, J.F.A.; Specker, E.J.; Romeo, C.M.; Coomber, B.L.; Uniacke, J. Hypoxia activates cadherin-22 synthesis via eIF4E2 to drive cancer cell migration, invasion and adhesion. Oncogene 2018, 37, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.; Wang, C.; Li, X.; Guan, F. Bisecting N-acetylglucosamine structures inhibit hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Front. Physiol. 2018, 9, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendt, J. Melatonin and the pineal gland: Influence on mammalian seasonal and circadian physiology. Rev. Reprod. 1998, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.V.; Mosser, E.A.; Oikonomou, G.; Prober, D.A. Melatonin is required for the circadian regulation of sleep. Neuron 2015, 85, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Scheer, F.A.; Van Montfrans, G.A.; van Someren, E.J.; Mairuhu, G.; Buijs, R.M. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Cagnacci, A.; Kräuchi, K.; Wirz-Justice, A.; Volpe, A. Homeostatic versus circadian effects of melatonin on core body temperature in humans. J. Biol. Rhythms 1997, 12, 509–517. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J.; Poeggler, B. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- D Mediavilla, M.; J Sanchez-Barcelo, E.; X Tan, D.; Manchester, L.; J Reiter, R. Basic mechanisms involved in the anti-cancer effects of melatonin. Curr. Med. Chem. 2010, 17, 4462–4481. [Google Scholar] [CrossRef]
- Lee, S.; Hallis, S.P.; Jung, K.A.; Ryu, D.; Kwak, M.K. Impairment of HIF-1α-mediated metabolic adaption by NRF2-silencing in breast cancer cells. Redox. Biol. 2019, 24, 101210. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.M.; Antolin, I.; Puente-Moncada, N.; Suarez, S.; Gomez-Lobo, M.; Rodriguez, C.; Martin, V. Melatonin cytotoxicity is associated to warburg effect inhibition in ewing sarcoma cells. PLoS ONE 2015, 10, e0135420. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.S.; Martins, A.D.; Rato, L.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Melatonin alters the glycolytic profile of Sertoli cells: Implications for male fertility. Mol. Hum. Reprod. 2014, 20, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.R.; Liu, H.B.; Dai Chen, Y.; Sha, Y.; Ma, Q.; Zhu, P.J.; Mu, Y. Melatonin attenuates myocardial ischemia/reperfusion injury by inhibiting autophagy via an AMPK/mTOR signaling pathway. Cell Physiol. Biochem. 2018, 47, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.A.; Shyu, K.G.; Luo, Y.; Magner, M.; Tio, R.A.; Jiang, C.; Goldberg, M.A.; Akita, G.Y.; Gregory, R.J.; Isner, J.M. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation 2000, 102, 2255–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megdal, S.P.; Kroenke, C.H.; Laden, F.; Pukkala, E.; Schernhammer, E.S. Night work and breast cancer risk: A systematic review and meta-analysis. Eur. J. Cancer 2005, 41, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J. Increased breast cancer risk among women who work predominantly at night. Epidemiology 2001, 12, 74–77. [Google Scholar] [CrossRef]
- Chiche, J.; Brahimi-Horn, M.C.; Pouysségur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.Y.; Lucas, J.E.; Schroeder, T.; Mori, S.; Wu, J.; Nevins, J.; Dewhirst, M.; West, M.; Chi, J.T. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008, 4, e1000293. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Tang, D.; Liu, N.; Xiong, W.; Huang, H.; Li, Y.; Ma, Z.; Zhao, H.; Chen, P.; Qi, X.; et al. Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab. 2017, 25, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Otálora, B.B.; Madrid, J.A.; Alvarez, N.; Vicente, V.; Rol, M.A. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J. Pineal Res. 2008, 44, 307–315. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-J.; Seo, E.-B.; Kwon, S.-H.; Lee, S.-H.; Kim, S.-K.; Park, S.K.; Kim, K.; Park, S.; Park, I.-C.; Park, J.-W.; et al. Extracellular Acidosis Promotes Metastatic Potency via Decrease of the BMAL1 Circadian Clock Gene in Breast Cancer. Cells 2020, 9, 989. https://doi.org/10.3390/cells9040989
Kwon Y-J, Seo E-B, Kwon S-H, Lee S-H, Kim S-K, Park SK, Kim K, Park S, Park I-C, Park J-W, et al. Extracellular Acidosis Promotes Metastatic Potency via Decrease of the BMAL1 Circadian Clock Gene in Breast Cancer. Cells. 2020; 9(4):989. https://doi.org/10.3390/cells9040989
Chicago/Turabian StyleKwon, Yong-Jin, Eun-Bi Seo, Sun-Ho Kwon, Song-Hee Lee, Seul-Ki Kim, Sang Ki Park, Kyungjin Kim, SaeGwang Park, In-Chul Park, Jong-Wan Park, and et al. 2020. "Extracellular Acidosis Promotes Metastatic Potency via Decrease of the BMAL1 Circadian Clock Gene in Breast Cancer" Cells 9, no. 4: 989. https://doi.org/10.3390/cells9040989
APA StyleKwon, Y. -J., Seo, E. -B., Kwon, S. -H., Lee, S. -H., Kim, S. -K., Park, S. K., Kim, K., Park, S., Park, I. -C., Park, J. -W., & Ye, S. -K. (2020). Extracellular Acidosis Promotes Metastatic Potency via Decrease of the BMAL1 Circadian Clock Gene in Breast Cancer. Cells, 9(4), 989. https://doi.org/10.3390/cells9040989