RNA Interference Screening Identifies Novel Roles for RhoBTB1 and RhoBTB3 in Membrane Trafficking Events in Mammalian Cells
Abstract
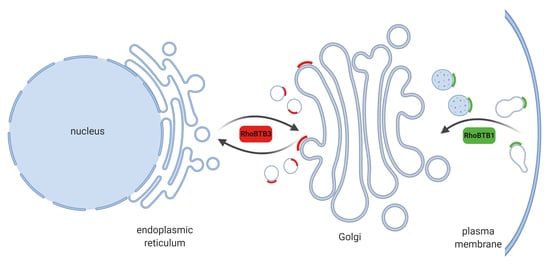
:1. Introduction
2. Materials and Methods
2.1. Antibodies and siRNA Molecules
2.2. DNA Plasmids
2.3. Cell Culture and Transfections
2.4. Immunofluorescence
2.5. Confocal Image Acquisition and Analysis
2.6. Polar Distribution Score Analysis
2.7. Live Cell Imaging Experiments and Analysis
2.8. Total RNA Extraction, cDNA Synthesis, and Real-Time Quantitative PCR
2.9. Shiga-Like Toxin-1 B Subunit and Transferrin Uptake Assays
2.10. Protein Extraction and Western Blotting
3. Results
3.1. Depletion of Several Rho GTPase Proteins Affects Golgi Complex Morphology
3.2. Cdc42 and RhoBTB3 Depletion Causes Changes to p24-YFP Carriers in the Early Secretory Pathway
3.3. RhoBTB3 Localises to Transport Carriers in the Early Secretory Pathway
3.4. RhoBTB1 Is Important for Retrograde Transport of the B Subunit of Shiga-Like Toxin-1
3.5. RhoBTB1 Protein Levels Affect Endosomal and Lysosomal Morphology
3.6. RhoBTB1 Depletion Affects Transferrin Uptake and Transport
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anitei, M.; Hoflack, B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat. Cell Biol. 2011, 14, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The Mechanisms of Vesicle Budding and Fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Brandizzi, F.; Barlowe, C. Organization of the ER-Golgi interface for membrane traffic control. Nat. Rev. Mol. Cell Biol. 2013, 14, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.A.; Schekman, R. COPII—A flexible vesicle formation system. Curr. Opin. Cell Biol. 2013, 25, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Navarro, N.; Miller, E. Protein sorting at the ER-Golgi interface. J. Cell Biol. 2016, 215, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Duleh, S.N.; Welch, M.D. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton 2010, 67, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Zilberman, Y.; Alieva, N.O.; Miserey-Lenkei, S.; Lichtenstein, A.; Kam, Z.; Sabanay, H.; Bershadsky, A. Involvement of the Rho-mDia1 pathway in the regulation of Golgi complex architecture and dynamics. Mol. Biol. Cell 2011, 22, 2900–2911. [Google Scholar] [CrossRef]
- Braun, A.C.; Hendrick, J.; Eisler, S.A.; Schmid, S.; Hausser, A.; Olayioye, M.A. The Rho-specific GAP protein DLC3 coordinates endocytic membrane trafficking. J. Cell Sci. 2015, 128, 1386–1399. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Ramiro, B.; García-Weber, D.; Barroso, S.; Feito, J.; Ortega, M.C.; Cernuda-Morollón, E.; Reglero-Real, N.; Fernández-Martín, L.; Durán, M.C.; Alonso, M.A.; et al. RhoB controls endothelial barrier recovery by inhibiting Rac1 trafficking to the cell border. J. Cell Biol. 2016, 213, 385–402. [Google Scholar] [CrossRef] [Green Version]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulou, M.; Hall, A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridley, A.J. Rho GTPase signalling in cell migration. Curr. Opin. Cell Biol. 2015, 36, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhan, H.; Hsu, V.W. Cdc42 and Cellular Polarity: Emerging Roles at the Golgi. Trends Cell Biol. 2016, 26, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardakheh, F.K.; Self, A.; Marshall, C.J. RHO binding to FAM65A regulates Golgi reorientation during cell migration. J. Cell Sci. 2016, 129, 4466–4479. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.; Saffrich, R.; Grummt, M.; Gournier, H.; Rybin, V.; Rubino, M.; Auvinen, P.; Lütcke, A.; Parton, R.G.; Zerial, M. Endosome dynamics regulated by a Rho protein. Nature 1996, 384, 427–432. [Google Scholar] [CrossRef]
- Murphy, C.; Saffrich, R.; Olivo-Marin, J.C.; Giner, A.; Ansorge, W.; Fotsis, T.; Zerial, M. Dual function of rhoD in vesicular movement and cell motility. Eur. J. Cell Biol. 2001, 80, 391–398. [Google Scholar] [CrossRef]
- Gasman, S.; Kalaidzidis, Y.; Zerial, M. RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat. Cell Biol. 2003, 5, 195–204. [Google Scholar] [CrossRef]
- Lu, A.; Pfeffer, S.R. Golgi-associated RhoBTB3 targets cyclin E for ubiquitylation and promotes cell cycle progression. J. Cell Biol. 2013, 203, 233–250. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, C.M.; Mellor, H. The tumor suppressor RhoBTB1 controls Golgi integrity and breast cancer cell invasion through METTL7B. BMC Cancer 2017, 17, 145. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.J.; Erickson, J.W.; Lin, R.; Cerione, R.A. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 2000, 405, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Fucini, R.V.; Lacomis, L.; Erdjument-Bromage, H.; Tempst, P.; Stamnes, M. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J. Cell Biol. 2005, 169, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Yang, J.-S.; Schmider, A.B.; Soberman, R.J.; Hsu, V.W. Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature 2015, 521, 529–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, J.C.; Nilsson, T.; Pepperkok, R. Biogenesis of tubular ER-to-Golgi transport intermediates. Mol. Biol. Cell 2006, 17, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girod, A.; Storrie, B.; Simpson, J.C.; Johannes, L.; Goud, B.; Roberts, L.M.; Lord, J.M.; Nilsson, T.; Pepperkok, R. Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1999, 1, 423–430. [Google Scholar] [CrossRef]
- Wada, I.; Rindress, D.; Cameron, P.H.; Ou, W.J.; Doherty, J.J., 2nd; Louvard, D.; Bell, A.W.; Dignard, D.; Thomas, D.Y.; Bergeron, J.J. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 1991, 266, 19599–19610. [Google Scholar]
- Stamnes, M.A.; Craighead, M.W.; Hoe, M.H.; Lampen, N.; Geromanos, S.; Tempst, P.; Rothman, J.E. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA 1995, 92, 8011–8015. [Google Scholar] [CrossRef] [Green Version]
- Rojo, M.; Pepperkok, R.; Emery, G.; Kellner, R.; Stang, E.; Parton, R.G.; Gruenberg, J. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J. Cell Biol. 1997, 139, 1119–1135. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Cantizano, N.; Montesinos, J.C.; Bernat-Silvestre, C.; Marcote, M.J.; Aniento, F. p24 family proteins: Key players in the regulation of trafficking along the secretory pathway. Protoplasma 2016, 253, 967–985. [Google Scholar] [CrossRef]
- Simpson, J.C.; Joggerst, B.; Laketa, V.; Verissimo, F.; Cetin, C.; Erfle, H.; Bexiga, M.G.; Singan, V.R.; Hériché, J.K.; Neumann, B.; et al. Genome-wide RNAi screening identifies human proteins with a regulatory function in the early secretory pathway. Nat. Cell Biol. 2012, 14, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Blom, M.; Reis, K.; Nehru, V.; Blom, H.; Gad, A.K.; Aspenström, P. RhoD is a Golgi component with a role in anterograde protein transport from the ER to the plasma membrane. Exp. Cell Res. 2015, 333, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; Matas, O.B.; Martınez-Menárguez, J.A.; Mato, E.; Durán, J.M.; Ballesta, J.; Way, M.; Egea, G. Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N-WASP. Mol. Biol. Cell 2002, 13, 866–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, E.J.; Calero, M.; Sridevi, K.; Pfeffer, S.R. RhoBTB3: A Rho GTPase-Family ATPase Required for Endosome to Golgi Transport. Cell 2009, 137, 938–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hehnly, H.; Longhini, K.M.; Chen, J.-L.; Stamnes, M. Retrograde Shiga toxin trafficking is regulated by ARHGAP21 and Cdc42. Mol. Biol. Cell 2009, 20, 4303–4312. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Grant, B.D. A TOCA/CDC-42/PAR/WAVE functional module required for retrograde endocytic recycling. Proc. Natl. Acad. Sci. USA 2015, 112, E1443–E1452. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Rivero, F. Atypical Rho GTPases of the RhoBTB Subfamily: Roles in Vesicle Trafficking and Tumorigenesis. Cells 2016, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Matthys, A.; Van Craenenbroeck, K.; Lintermans, B.; Haegeman, G.; Vanhoenacker, P. RhoBTB3 interacts with the 5-HT7a receptor and inhibits its proteasomal degradation. Cell. Signal. 2012, 24, 1053–1063. [Google Scholar] [CrossRef]
- Berger, M.; Riley, D.R.; Lutz, J.; Khalil, J.S.; Aburima, A.; Naseem, K.M.; Rivero, F. Alterations in Platelet Alpha-Granule Secretion and Adhesion on Collagen under Flow in Mice Lacking the Atypical Rho GTPase RhoBTB3. Cells 2019, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Haga, R.B.; Garg, R.; Collu, F.; Borda D’Agua, B.; Menéndez, S.T.; Colomba, A.; Fraternali, F.; Ridley, A.J. RhoBTB1 interacts with ROCKs and inhibits invasion. Biochem. J. 2019, 476, 2499–2514. [Google Scholar] [CrossRef] [Green Version]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Berthold, J.; Schenková, K.; Ramos, S.; Miura, Y.; Furukawa, M.; Aspenström, P.; Rivero, F. Characterization of RhoBTB-dependent Cul3 ubiquitin ligase complexes—Evidence for an autoregulatory mechanism. Exp. Cell Res. 2008, 314, 3453–3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huotari, J.; Meyer-Schaller, N.; Hubner, M.; Stauffer, S.; Katheder, N.; Horvath, P.; Mancini, R.; Helenius, A.; Peter, M. Cullin-3 regulates late endosome maturation. Proc. Natl. Acad. Sci. USA 2012, 109, 823–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gschweitl, M.; Ulbricht, A.; Barnes, C.A.; Enchev, R.I.; Stoffel-Studer, I.; Meyer-Schaller, N.; Huotari, J.; Yamauchi, Y.; Greber, U.F.; Helenius, A.; et al. A SPOPL/cullin-3 ubiquitin ligase complex regulates endocytic trafficking by targeting EPS15 at endosomes. eLife 2016, 5, e13841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, M.; Tanigawa, K.; Sakaue, T.; Hiyoshi, H.; Kubota, E.; Joh, T.; Watanabe, Y.; Taguchi, T.; Higashiyama, S. Cullin-3 and its adaptor protein ANKFY1 determine the surface level of integrin β1 in endothelial cells. Biol. Open 2017, 6, 1707–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Kranjc, T.; Mysior, M.M.; Simpson, J.C. RNA Interference Screening Identifies Novel Roles for RhoBTB1 and RhoBTB3 in Membrane Trafficking Events in Mammalian Cells. Cells 2020, 9, 1089. https://doi.org/10.3390/cells9051089
Long M, Kranjc T, Mysior MM, Simpson JC. RNA Interference Screening Identifies Novel Roles for RhoBTB1 and RhoBTB3 in Membrane Trafficking Events in Mammalian Cells. Cells. 2020; 9(5):1089. https://doi.org/10.3390/cells9051089
Chicago/Turabian StyleLong, Maeve, Tilen Kranjc, Margaritha M. Mysior, and Jeremy C. Simpson. 2020. "RNA Interference Screening Identifies Novel Roles for RhoBTB1 and RhoBTB3 in Membrane Trafficking Events in Mammalian Cells" Cells 9, no. 5: 1089. https://doi.org/10.3390/cells9051089
APA StyleLong, M., Kranjc, T., Mysior, M. M., & Simpson, J. C. (2020). RNA Interference Screening Identifies Novel Roles for RhoBTB1 and RhoBTB3 in Membrane Trafficking Events in Mammalian Cells. Cells, 9(5), 1089. https://doi.org/10.3390/cells9051089