Physical Activity and Brain Health
Abstract
:1. Introduction
2. Brain Plasticity, Adult Neurogenesis, and Physical Activity
2.1. Brain-Derived Neurotrophic Factor (BDNF)
2.2. microRNAs and Exercise
2.3. Genes Involved in Mitochondrial and Lysosomal Biogenesis
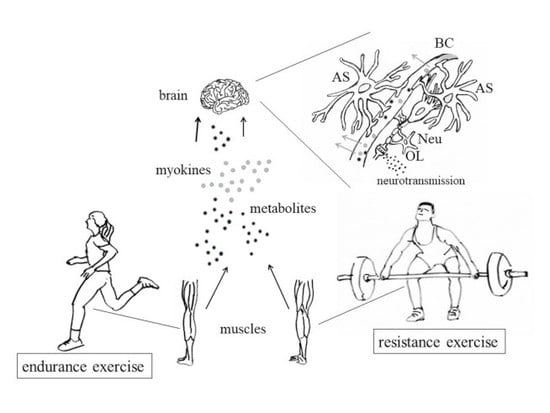
3. Muscle Contraction and Production of Myokines
3.1. Muscle Contraction and Gene Regulation
3.2. Release of Myokines and Metabolites by Contracting Muscles
3.2.1. BDNF and Cathepsin-B (CTSB)
3.2.2. FGF21 and Irisin/FNDC5
3.2.3. Cytokines Released by Muscles
3.2.4. Lactate
3.2.5. Extracellular Vesicles (EVs)
4. A Few Examples of Exercise Effects on Neurodegeneration: Studies on Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease, and Multiple Sclerosis
4.1. Alzheimer’s Disease (AD)
4.2. Parkinson’s Disease (PD)
4.3. Huntington’s Disease (HD)
4.4. Multiple Sclerosis (MS)
5. Exercise-Dependent Production of Dopamine, Endocannabinoids, and Opioids: Effects on Mood, Analgesia, and Happiness
5.1. Dopamine
5.2. Opioids, Endocannabinoids, Analgesia, and the “Runner’s High”
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panegyres, K.P.; Panegyres, P.K. The Ancient Greek discovery of the nervous system: Alcmaeon, Praxagoras and Herophilus. J. Clin. Neurosci. 2016, 29, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Wills, A. Herophilus, Erasistratus, and the birth of neuroscience. Lancet 1999, 354, 1719–1720. [Google Scholar] [CrossRef]
- Russo, L. The Forgotten Revolution; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; ISBN 3-540-20068-1. [Google Scholar]
- Von Staden, H. Herophilus: The Art of Medicine in Early Alexandria, 1st ed.; Cambridge University Press: Cambridge, UK, 2008; ISBN 9780521041782. [Google Scholar]
- Reveron, R.R. Herophilus and Erasistratus, pioneers of human anatomical dissection. Vesalius 2014, 20, 55–58. [Google Scholar] [PubMed]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramble, D.; Lieberman, D.E. Endurance running and the evolution of Homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Polk, J.D. Linking brains and brawn: Exercise and the evolution of human neurobiology. Proc. Biol. Sci. 2013, 280, 20122250. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.; Polk, J.D. BDNF, endurance activity, and mechanisms underlying the evolution of hominin brains. Am. J. Phys. Anthropol. 2019, 168 (Suppl. 67), 47–62. [Google Scholar] [CrossRef] [PubMed]
- Wikgren, J.; Mertikas, G.G.; Raussi, P.; Tirkkonen, R.; Äyräväinen, L.; Pelto-Huikko, M.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Selective breeding for endurance running capacity affects cognitive but not motor learning in rats. Physiol. Behav. 2012, 106, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Carrier, D.R. The Energetic Paradox of Human Running and Hominid Evolution. Curr. Anthropol. 1984, 25, 483–495. [Google Scholar] [CrossRef]
- Wheeler, P.E. The thermoregulatory advantages of hominid bipedalism in open equatorial environments: The contribution of increased convective heat loss and cutaneous evaporative cooling. J. Hum. Evol. 1991, 21, 107–115. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Wilkinson, D.M. Avoidance of overheating and selection for both hair loss and bipedality in hominins. Proc. Natl. Acad. Sci. USA 2011, 108, 20965–20969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, D.L., Jr.; Zhao, J.L.; Wu, Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J. Appl. Physiol. 2009, 107, 1438–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.C.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J. Invest. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. Available online: https://health.gov/paguidelines/second-edition/ (accessed on 1 June 2019).
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Sosner, P.; Guiraud, T.; Gremeaux, V.; Arvisais, D.; Herpin, D.; Bosquet, L. The ambulatory hypotensive effect of aerobic training: A reappraisal through a meta-analysis of selected moderators. Scand. J. Med. Sci. Sports 2017, 27, 327–341. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. 2018 Physical Activity Guidelines Advisory Committee. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Rêgo, M.L.; Cabral, D.A.; Costa, E.C.; Fontes, E.B. Physical Exercise for Individuals with Hypertension: It Is Time to Emphasize its Benefits on the Brain and Cognition. Clin. Med. Insights Cardiol. 2019, 13. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Parducci, P.; Livingston, J.; Taylor, B.A. A Systematically Assembled Signature of Genes to be Deep-Sequenced for Their Associations with the Blood Pressure Response to Exercise. Genes 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Bajer, B.; Vlcek, M.; Galusova, A.; Imrich, R.; Penesova, A. Exercise associated hormonal signals as powerful determinants of an effective fat mass loss. Endocr. Regul. 2015, 49, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.-C.; Liaw, Y.-P.; Lan, T.H. Physical Activity Might Reduce the Adverse Impacts of the FTO Gene Variant rs3751812 on the Body Mass Index of Adults in Taiwan. Genes 2019, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Mooren, F.C.; Pilat, C. The Immunomodulatory Effects of Physical Activity. Curr. Pharm. Des. 2016, 22, 3730–3748. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013, 9, CD004366. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- Cavalcante, P.A.M.; Gregnani, M.F.; Henrique, J.S.; Ornellas, F.H.; Araújo, R.C. Aerobic but not Resistance Exercise Can Induce Inflammatory Pathways via Toll-Like 2 and 4: A Systematic Review. Sports Med. Open 2017, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.; Soligard, T.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef]
- Ch’ng, T.H.; Uzgil, B.; Lin, P.; Avliyakulov, N.K.; O’Dell, T.J.; Martin, K.C. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell 2012, 150, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.F.; Lim, W.L.; Ch’ng, T.H. Activity-dependent synapse to nucleus signaling. Neurobiol. Learn. Mem. 2017, 138, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Herbst, W.A.; Martin, K.C. Regulated transport of signaling proteins from synapse to nucleus. Curr. Opin. Neurobiol. 2017, 45, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Marcello, E.; Di Luca, M.; Gardoni, F. Synapse-to-nucleus communication: From developmental disorders to Alzheimer’s disease. Curr. Opin. Neurobiol. 2018, 48, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Parra-Damas, A.; Saura, C.A. Synapse-to-Nucleus Signaling in Neurodegenerative and Neuropsychiatric Disorders. Biol. Psychiatry 2019, 86, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.N. Understanding calcium waves and sparks in central neurons. Nat. Rev. Neurosci. 2012, 13, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bading, H. Nuclear calcium signalling in the regulation of brain function. Nat. Rev. Neurosci. 2013, 14, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Proepper, C.; Johannsen, S.; Liebau, S.; Dahl, J.; Vaida, B.; Bockmann, J.; Kreutz, M.R.; Gundelfinger, E.D.; Boeckers, T.M. Abelson interacting protein 1 (Abi-1) is essential for dendrite morphogenesis and synapse formation. EMBO J. 2007, 26, 1397–1409. [Google Scholar] [CrossRef] [Green Version]
- Spilker, C.; Nullmeier, S.; Grochowska, K.M.; Schumacher, A.; Butnaru, I.; Macharadze, T.; Gomes, G.M.; Yuanxiang, P.; Bayraktar, G.; Rodenstein, C.; et al. A Jacob/Nsmf Gene Knockout Results in Hippocampal Dysplasia and Impaired BDNF Signaling in Dendritogenesis. PLoS Genet. 2016, 12, e1005907. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Regulation of mRNA transport, localization and translation in the nervous system of mammals (Review). Int. J. Mol. Med. 2014, 33, 747–762. [Google Scholar] [CrossRef] [Green Version]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Das, G.D. Postnatal neurogenesis in the guinea-pig. Nature 1967, 214, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.S.; Hinds, J.W. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science 1977, 197, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.S.; Bell, D.H. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J. Neurosci. 1984, 4, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, B.B.; Trice, J.E. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp. Brain Res. 1988, 72, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Woolley, C.S.; McEwen, B.S.; Gould, E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 1993, 56, 337–344. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Aimone, J.B.; Gage, F.H. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010, 11, 339–350. [Google Scholar] [CrossRef]
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021766. [Google Scholar] [CrossRef]
- Kim, S.; Dede, A.J.; Hopkins, R.O.; Squire, L.R. Memory, scene construction, and the human hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, 4767–4772. [Google Scholar] [CrossRef] [Green Version]
- Gould, E.; Beylin, A.; Tanapat, P.; Reeves, A.; Shors, T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999, 2, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H. Exercise and the brain: Something to chew on. Trends Neurosci. 2009, 32, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S.J.; Asare, M. Physical activity and mental health in children and adolescents: A review of reviews. Br. J. Sports Med. 2011, 45, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Niederer, I.; Kriemler, S.; Gut, J.; Hartmann, T.; Schindler, C.; Barral, J.; Puder, J.J. Relationship of aerobic fitness and motor skills with memory and attention in preschoolers (Ballabeina): A cross-sectional and longitudinal study. BMC Pediatr. 2011, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Hopkins, J. Effect of Aerobic Exercise on Cognition, Academic Achievement, and Psychosocial Function in Children: A Systematic Review of Randomized Control Trials. Prev. Chronic Dis. 2013, 10, E174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.L.; Ma, X.T.; Wang, J.J.; Liu, H.; Chen, Y.F.; Yang, Y. Physical exercise induces hippocampal neurogenesis and prevents cognitive decline. Behav. Brain Res. 2017, 317, 332–339. [Google Scholar] [CrossRef]
- Schmidt-Kassow, M.; Zink, N.; Mock, J.; Thiel, C.; Vogt, L.; Abel, C.; Kaiser, J. Treadmill walking during vocabulary encoding improves verbal long-term memory. Behav. Brain Funct. 2014, 10, 24. [Google Scholar] [CrossRef]
- Suwabe, K.; Hyodo, K.; Byun, K.; Ochi, G.; Yassa, M.A.; Soya, H. Acute moderate exercise improves mnemonic discrimination in young adults. Hippocampus 2017, 27, 229–234. [Google Scholar] [CrossRef]
- Rodriguez-Ayllon, M.; Cadenas-Sánchez, C.; Estévez-López, F.; Muñoz, N.E.; Mora-Gonzalez, J.; Migueles, J.H.; Molina-García, P.; Henriksson, H.; Mena-Molina, A.; Martínez-Vizcaíno, V.; et al. Role of Physical Activity and Sedentary Behavior in the Mental Health of Preschoolers, Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 1383–1410. [Google Scholar] [CrossRef] [PubMed]
- Niederer, I.; Kriemler, S.; Zahner, L.; Bürgi, F.; Ebenegger, V.; Hartmann, T.; Meyer, U.; Schindler, C.; Nydegger, A.; Marques-Vidal, P.; et al. Influence of a lifestyle intervention in preschool children on physiological and psychological parameters (Ballabeina): Study design of a cluster randomized controlled trial. BMC Public Health 2009, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Léger, L.A.; Mercier, D.; Gadoury, C.; Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sports Sci. 1988, 6, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lezi, E.; Burns, J.M.; Swerdlow, R.H. Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 2014, 35, 2574–2583. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ding, Y.H.; Rafols, J.A.; Lai, Q.; McAllister, J.P., 2nd; Ding, Y. Increased astrocyte proliferation in rats after running exercise. Neurosci. Lett. 2005, 386, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Saur, L.; Baptista, P.P.; de Senna, P.N.; Paim, M.F.; do Nascimento, P.; Ilha, J.; Bagatini, P.B.; Achaval, M.; Xavier, L.L. Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct. Funct. 2014, 219, 293–302. [Google Scholar] [CrossRef]
- Loprinzi, P.D. The role of astrocytes on the effects of exercise on episodic memory function. Physiol. Int. 2019, 106, 21–28. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus 2009, 19, 962–972. [Google Scholar] [CrossRef] [Green Version]
- Molnar, E. Long-term potentiation in cultured hippocampal neurons. Semin. Cell. Dev. Biol. 2011, 22, 506–513. [Google Scholar] [CrossRef]
- Horvath, S.; Pirazzini, C.; Bacalini, M.G.; Gentilini, D.; Di Blasio, A.M.; Delledonne, M.; Mari, D.; Arosio, B.; Monti, D.; Passarino, G.; et al. Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring. Aging 2015, 7, 1159–1170. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, L.; Lenart, A.; Tan, Q.; Vaupel, J.W.; Aviv, A.; McGue, M.; Christensen, K. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell 2016, 15, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, J.R.; Dudley-Javoroski, S.; Shields, R.K. Precision Physical Therapy: Exercise, the Epigenome, and the Heritability of Environmentally Modified Traits. Phys. Ther. 2018, 98, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; James, L.; Hussey, B.; Wadley, A.J.; Lindley, M.R.; Mastana, S.S. Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics 2019, 14, 294–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, A.; Koliamitra, C.; Bauer, C.J.; Schier, R.; Schweiger, M.R.; Bloch, W.; Zimmer, P. Impact of Acute Aerobic Exercise on Genome-Wide DNA-Methylation in Natural Killer Cells-A Pilot Study. Genes 2019, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H. Neurogenesis and exercise: Past and future directions. Neuromol. Med. 2008, 10, 128–140. [Google Scholar] [CrossRef]
- Lista, I.; Sorrentino, G. Biological mechanisms of physical activity in preventing cognitive decline. Cell. Mol. Neurobiol. 2010, 30, 493–503. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Denham, J. Exercise and epigenetic inheritance of disease risk. Acta Physiol. 2018, 222, e12881. [Google Scholar] [CrossRef]
- Biterge, B.; Schneider, R. Histone variants: Key players of chromatin. Cell Tissue Res. 2014, 356, 457–466. [Google Scholar] [CrossRef]
- Li, G.; Zhu, P. Structure and organization of chromatin fiber in the nucleus. FEBS Lett. 2015, 589, 2893–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.; Henikoff, S. Nucleosome dynamics during chromatin remodeling in vivo. Nucleus 2016, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Di Liegro, I. H1.0 Linker Histone as an Epigenetic Regulator of Cell Proliferation and Differentiation. Genes 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Zhou, M.M. Structure and mechanisms of lysine methylation recognition by the chromodomain in gene transcription. Biochemistry 2011, 50, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.; Brehm, A. Chromatin regulation: How complex does it get? Epigenetics 2014, 9, 1485–1495. [Google Scholar] [CrossRef] [Green Version]
- Ausió, J.; Georgel, P.T. MeCP2 and CTCF: Enhancing the cross-talk of silencers. Biochem. Cell Biol. 2017, 95, 593–608. [Google Scholar] [CrossRef]
- Jain, A.K.; Barton, M.C. Bromodomain Histone Readers and Cancer. J. Mol. Biol. 2017, 429, 2003–2010. [Google Scholar] [CrossRef]
- Han, P.; Chang, C.P. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015, 12, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Dimmeler, S. Exercise controls non-coding RNAs. Cell Metab. 2015, 21, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Alibegovic, A.C.; Sonne, M.P.; Højbjerre, L.; Bork-Jensen, J.; Jacobsen, S.; Nilsson, E.; Faerch, K.; Hiscock, N.; Mortensen, B.; Friedrichsen, M.; et al. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E752–E763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.W.; Chen, L. Epigenetic Regulation of BDNF Gene during Development and Diseases. Int. J. Mol. Sci. 2017, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- De Assis, G.G.; Gasanov, E.V.; de Sousa, M.B.C.; Kozacz, A.; Murawska-Cialowicz, E. Brain derived neutrophic factor, a link of aerobic metabolism to neuroplasticity. J. Physiol. Pharmacol. 2018, 69, 351–358. [Google Scholar] [CrossRef]
- Nofuji, Y.; Suwa, M.; Sasaki, H.; Ichimiya, A.; Nishichi, R.; Kumagai, S. Different circulating brain-derived neurotrophic factor responses to acute exercise between physically active and sedentary subjects. J. Sports Sci. Med. 2012, 11, 83–88. [Google Scholar] [PubMed]
- Hung, C.L.; Tseng, J.W.; Chao, H.H.; Hung, T.M.; Wang, H.S. Effect of Acute Exercise Mode on Serum Brain-Derived Neurotrophic Factor (BDNF) and Task Switching Performance. J. Clin. Med. 2018, 7, 301. [Google Scholar] [CrossRef]
- Saucedo Marquez, C.M.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol. (1985) 2015, 119, 1363–1373. [Google Scholar] [CrossRef] [Green Version]
- Etnier, J.L.; Wideman, L.; Labban, J.D.; Piepmeier, A.T.; Pendleton, D.M.; Dvorak, K.K.; Becofsky, K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 2016, 38, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Håkansson, K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.C.; Mohammed, A.K. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J. Alzheimers Dis. 2017, 55, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.-Y. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp. Gerontol. 2018, 104, 60–65. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, A.; Solana, E.; Corpas, R.; Bartrés-Faz, D.; Pallàs, M.; Vina, J.; Sanfeliu, C.; Gomez-Cabrera, M.C. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin, B. Sci. Rep. 2019, 9, 3337. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, K.P.M.; de Oliveira Segundo, V.H.; de Medeiros, G.C.B.S.; de Sousa Mata, Á.N.; García, D.Á.; de Carvalho Leitão, J.C.G.; Knackfuss, M.I.; Piuvezam, G. Effects of exercise on the levels of BDNF and executive function in adolescents: A protocol for systematic review and meta-analysis. Medicine 2019, 98, e16445. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Vaynman, S.; Ying, Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur. J. Neurosci. 2008, 28, 2278–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Zhuang, Y.; Feng, J.; Ying, Z.; Fan, G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci. 2011, 33, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ieraci, A.; Mallei, A.; Musazzi, L.; Popoli, M. Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 2015, 25, 1380–1392. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Li, Y.W. Focused microwave irradiation-assisted immunohistochemistry to study effects of ketamine on phospho-ERK expression in the mouse brain. Brain Res. 2017, 1670, 86–95. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, A.; Wang, Y.; Hu, S.; Zhang, R.; Qian, S. Genome-wide identification of brain miRNAs in response to high-intensity intermittent swimming training in Rattus norvegicus by deep sequencing. BMC Mol. Biol. 2019, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Beclin, C.; Follert, P.; Stappers, E.; Barral, S.; Coré, N.; de Chevigny, A.; Magnone, V.; Lebrigand, K.; Bissels, U.; Huylebroeck, D.; et al. miR-200 family controls late steps of postnatal forebrain neurogenesis via Zeb2 inhibition. Sci. Rep. 2016, 6, 35729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Singh, P.; Jauhari, A.; Singh, T.; Khan, F.; Pant, A.B.; Parmar, D.; Yadav, S. Critical role of the miR-200 family in regulating differentiation and proliferation of neurons. J. Neurochem. 2015, 133, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Jauhari, A.; Yadav, S. MiR-34 and MiR-200: Regulator of Cell Fate Plasticity and Neural Development. Neuromol. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.S.; Zakhary, L.; Choi, W.Y.; Caron, S.; Alvarez-Saavedra, E.; Miska, E.A.; McManus, M.; Harfe, B.; Giraldez, A.J.; Horvitz, H.R.; et al. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron 2008, 57, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Yoon, H.J.; Jeon, D.; Kang, K.M.; Park, K.H.; Bae, E.K.; Kim, M.; Lee, S.K.; et al. MicroRNAs induced during ischemic preconditioning. Stroke 2010, 41, 1646–1651. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, F.J.; Chang, Y.F.; Li, Y.S.; Liu, G.C.; Hong, Y.; Chen, H.L.; Xiyang, Y.B.; Bao, T.H. miR21 is Associated with the Cognitive Improvement Following Voluntary Running Wheel Exercise in TBI Mice. J. Mol. Neurosci. 2015, 57, 114–122. [Google Scholar] [CrossRef]
- Kou, X.; Li, J.; Liu, X.; Chang, J.; Zhao, Q.; Jia, S.; Fan, J.; Chen, N. Swimming attenuates d-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J. Appl. Physiol. 2017, 122, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Frenzel, M.; Rommelspacher, H.; Sugawa, M.D.; Dencher, N.A. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp. Gerontol. 2010, 45, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.T.; Brewer, G.J. Age-related deficiencies in complex I endogenous substrate availability and reserve capacity of complex IV in cortical neuron electron transport. Biochim. Biophys. Acta 2010, 1797, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Shen, L.; Hu, P.; Huang, R.; Cao, Y.; Deng, J.; Yuan, W.; Liu, D.; Yang, J.; Gu, H.; et al. Aging-associated mitochondrial DNA mutations alter oxidative phosphorylation machinery and cause mitochondrial dysfunctions. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Trushin, S.; Christensen, T.A.; Bachmeier, B.V.; Gateno, B.; Schroeder, A.; Yao, J.; Itoh, K.; Sesaki, H.; Poon, W.W.; et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci. Rep. 2016, 6, 18725. [Google Scholar] [CrossRef]
- Hara, Y.; Yuk, F.; Puri, R.; Janssen, W.G.; Rapp, P.R.; Morrison, J.H. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc. Natl. Acad. Sci. USA 2014, 111, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Bubber, P.; Haroutunian, V.; Fisch, G.; Blass, J.P.; Gibson, G.E. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Ann. Neurol. 2005, 57, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Palomo, G.M.; Manfredi, G. Exploring new pathways of neurodegeneration in ALS: The role of mitochondria quality control. Brain Res. 2015, 1607, 36–46. [Google Scholar] [CrossRef]
- Lee, K.S.; Huh, S.; Lee, S.; Wu, Z.; Kim, A.K.; Kang, H.Y.; Lu, B. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc. Natl. Acad. Sci. USA 2018, 115, E8844–E8853. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Callio, J.; Distefano, G.; O’Doherty, R.M.; Goodpaster, B.H.; Coen, P.M.; Chu, C.T. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp. Gerontol. 2017, 90, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Okutsu, M.; Zhang, M.; Greene, N.P.; Laker, R.C.; Breen, D.S.; Hoehn, K.L.; Yan, Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013, 27, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Bernardi, H.; Py, G.; Candau, R.B. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R956–R969. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, M.M.; Halling, J.F.; Møller, H.D.; Plomgaard, P.; Regenberg, B.; Ringholm, S.; Pilegaard, H. Regulation of apoptosis and autophagy in mouse and human skeletal muscle with aging and lifelong exercise training. Exp. Gerontol. 2018, 111, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Zhu, Y.; Li, Z.; Zhu, Y.T.; Wu, J.C.; Qin, Z.H.; Xiang, M.; Lin, F. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coupling mitogenesis and mitophagy for longevity. Autophagy 2015, 11, 1428–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, O.C.; Estébanez, B.; Martínez-Florez, S.; de Paz, J.A.; Cuevas, M.J.; González-Gallego, J. Mitochondrial Function and Mitophagy in the Elderly: Effects of Exercise. Oxid. Med. Cell. Longev. 2017, 2017, 2012798. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.M.; Moreli, M.L.; Tewari, S.; Benite-Ribeiro, S.A. The effect of exercise on skeletal muscle glucose uptake in type 2 diabetes: An epigenetic perspective. Metabolism 2015, 64, 1619–1628. [Google Scholar] [CrossRef]
- Rezapour, S.; Shiravand, M.; Mardani, M. Epigenetic changes due to physical activity. Biotechnol. Appl. Biochem. 2018, 65, 761–767. [Google Scholar] [CrossRef]
- Chaillou, T. Skeletal Muscle Fiber Type in Hypoxia: Adaptation to High-Altitude Exposure and Under Conditions of Pathological Hypoxia. Front. Physiol. 2018, 9, 1450. [Google Scholar] [CrossRef]
- Gundersen, K. Excitation-transcription coupling in skeletal muscle: The molecular pathways of exercise. Biol. Rev. Camb. Philos. Soc. 2011, 86, 564–600. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, R.; Konno, R.; Ohsawa, I.; Watanabe, A.; Kawano, F. Muscle type-specific RNA polymerase II recruitment during PGC-1α gene transcription after acute exercise in adult rats. J. Appl. Physiol. (1985) 2018, 125, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Vrbová, G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve 1999, 22, 666–677. [Google Scholar] [CrossRef]
- Pette, D. Training effects on the contractile apparatus. Acta Physiol. Scand. 1998, 162, 367–376. [Google Scholar] [CrossRef]
- Hughes, S.M.; Koishi, K.; Rudnicki, M.; Maggs, A.M. MyoD protein is differentially accumulated in fast and slow skeletal muscle fibres and required for normal fibre type balance in rodents. Mech. Dev. 1997, 61, 151–163. [Google Scholar] [CrossRef]
- Macharia, R.; Otto, A.; Valasek, P.; Patel, K. Neuromuscular junction morphology, fiber-type proportions, and satellite-cell proliferation rates are altered in MyoD(-/-) mice. Muscle Nerve 2010, 42, 38–52. [Google Scholar] [CrossRef]
- Parsons, S.A.; Millay, D.P.; Wilkins, B.J.; Bueno, O.F.; Tsika, G.L.; Neilson, J.R.; Liberatore, C.M.; Yutzey, K.E.; Crabtree, G.R.; Tsika, R.W.; et al. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J. Biol. Chem. 2004, 279, 26192–26200. [Google Scholar] [CrossRef]
- Oh, M.; Rybkin, I.I.; Copeland, V.; Czubryt, M.P.; Shelton, J.M.; van Rooij, E.; Richardson, J.A.; Hill, J.A.; De Windt, L.J.; Bassel-Duby, R.; et al. Calcineurin is necessary for the maintenance but not embryonic development of slow muscle fibers. Mol. Cell. Biol. 2005, 25, 6629–6638. [Google Scholar] [CrossRef]
- Rana, Z.A.; Gundersen, K.; Buonanno, A. Activity-dependent repression of muscle genes by NFAT. Proc. Natl. Acad. Sci. USA 2008, 105, 5921–5926. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, M.L.; Celona, B.; Black, B.L. NFATc1 controls skeletal muscle fiber type and is a negative regulator of MyoD activity. Cell Rep. 2014, 8, 1639–1648. [Google Scholar] [CrossRef]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The role of calcium ions, calmodulin and troponin in the regulation of phosphorylase kinase from rabbit skeletal muscle. Eur. J. Biochem. 1980, 111, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.; Honeycutt, T.; Blumenthal, D.K. The gamma-ubunit of Skeletal Muscle Phosphorylase Kinase Contains Two Noncontiguous Domains That Act in Concert to Bind Calmodulin. J. Biol. Chem. 1989, 264, 17156–17163. [Google Scholar] [PubMed]
- Sola-Penna, M.; Da, S.D.; Coelho, W.S.; Marinho-Carvalho, M.M.; Zancan, P. Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life 2010, 62, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Marxsen, J.H.; Stengel, P.; Doege, K.; Heikkinen, P.; Jokilehto, T.; Wagner, T.; Jelkmann, W.; Jaakkola, P.; Metzen, E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-α-prolyl-4-hydroxylases. Biochem. J. 2004, 381 Pt 3, 761–767. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the HIF transactivation domain: A hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Richeit, P.; Knaus, P.; Coirault, C. YAP-Mediated Mechanotransduction in Skeletal Muscle. Front. Physiol. 2016, 7, 41. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell. Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Imajo, M.; Miyatake, K.; Iimura, A.; Miyamoto, A.; Nishida, E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/b-catenin signalling. EMBO J. 2012, 31, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Lessard, S.J.; MacDonald, T.L.; Pathak, P.; Han, M.S.; Coffey, V.G.; Edge, J.; Rivas, D.A.; Hirshman, M.F.; Davis, R.J.; Goodyear, L.J. JNK regulates muscle remodeling via myostatin/SMAD inhibition. Nat. Commun. 2018, 9, 3030. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Boppart, M.D.; Dufresne, S.D.; Fielding, R.A.; Goodyear, L.J. Exercise stimulates c-Jun NH2 kinase activity and c-Jun transcriptional activity in human skeletal muscle. Biochem. Biophys. Res. Commun. 1998, 251, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Ugrinowitsch, C.; Libardi, C.A.; Jannig, P.R.; Hector, A.J.; McGlory, C.; Lixandrão, M.E.; Vechin, F.C.; Montenegro, H.; Tricoli, V.; et al. Resistance training in young men induces muscle transcriptome-wide changes associated with muscle structure and metabolism refining the response to exercise-induced stress. Eur. J. Appl. Physiol. 2018, 118, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Wessner, B.; Liebensteiner, M.; Nachbauer, W.; Csapo, R. Age-specific response of skeletal muscle extracellular matrix to acute resistance exercise: A pilot study. Eur. J. Sports Sci. 2019, 19, 354–364. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Fairlie, E.; Garnham, A.P.; Hargreaves, M. Exercise-induced histone modifications in human skeletal muscle. J. Physiol. 2009, 587 Pt 24, 5951–5958. [Google Scholar] [CrossRef]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetic adaptation to regular exercise in humans. Drug Discov. Today 2014, 19, 1015–1018. [Google Scholar] [CrossRef]
- King-Himmelreich, T.S.; Schramm, S.; Wolters, M.C.; Schmetzer, J.; Möser, C.V.; Knothe, C.; Resch, E.; Peil, J.; Geisslinger, G.; Niederberger, E. The impact of endurance exercise on global and AMPK gene-specific DNA methylation. Biochem. Biophys. Res. Commun. 2016, 474, 284–290. [Google Scholar] [CrossRef]
- Russell, A.P.; Lamon, S.; Boon, H.; Wada, S.; Güller, I.; Brown, E.L.; Chibalin, A.V.; Zierath, J.R.; Snow, R.J.; Stepto, N.; et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J. Physiol. 2013, 591, 4637–4653. [Google Scholar] [CrossRef]
- Silva, G.J.J.; Bye, A.; El Azzouzi, H.; Wisløff, U. MicroRNAs as Important Regulators of Exercise Adaptation. Prog. Cardiovasc. Dis. 2017, 60, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Scheele, C.; Yfanti, C.; Akerström, T.; Nielsen, A.R.; Pedersen, B.K.; Laye, M.J. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J. Physiol. 2010, 588 Pt 20, 4029–4037. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. S-Glutathionylation signaling in cell biology: Progress and prospects. Eur. J. Pharm. Sci. 2012, 46, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.A.; Duan, J.; Gaffrey, M.J.; Shukla, A.K.; Wang, L.; Bammler, T.K.; Qian, W.J.; Marcinek, D.J. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol. 2018, 17, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal muscle is an endocrine organ. J. Pharmacol. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1a, myokines and exercise. Bone 2015, 80, 115125. [Google Scholar] [CrossRef] [PubMed]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Matthews, V.B.; Astrom, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [Green Version]
- Ogborn, D.I.; Gardiner, P.F. Effects of exercise and muscle type on BDNF, NT-4/5, and TrKB expression in skeletal muscle. Muscle Nerve 2010, 41, 385–391. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Buck, M.R.; Karustis, D.G.; Day, N.A.; Honn, K.V.; Sloane, B.F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 1992, 282, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linebaugh, B.E.; Sameni, M.; Day, N.A.; Sloane, B.F.; Keppler, D. Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur. J. Biochem. 1999, 264, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.O.; Tadinada, S.M.; Zasadny, F.M.; Oliveira, K.J.; Pires, K.M.P.; Olvera, A.; Jeffers, J.; Souvenir, R.; Mcglauflin, R.; Seei, A.; et al. OPA1 deficiency promotes secretion of FGF21 from muscle that prevents obesity and insulin resistance. EMBO J. 2017, 36, 2126–2145. [Google Scholar] [CrossRef] [PubMed]
- BonDurant, L.D.; Potthoff, M.J. Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu. Rev. Nutr. 2018, 38, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef] [Green Version]
- Kuro-O, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Baktir, M.A.; Srivatsan, M.; Salehi, A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front. Cell. Neurosci. 2014, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Semple, B.D.; Kossmann, T.; Morganti-Kossmann, M.C. Role of chemokines in CNS health and pathology: A focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010, 30, 459–473. [Google Scholar] [CrossRef]
- Pan, W.; Wu, X.; He, Y.; Hsuchou, H.; Huang, E.Y.; Mishra, P.K.; Kastin, A.J. Brain interleukin-15 in neuroinflammation and behavior. Neurosci. Biobehav. Rev. 2013, 37, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen, J.; Bergersen, L.H.; Wrann, C.D.; Selbæk, G.; Kivipelto, M.; Moreira, J.B.N.; et al. Are the neuroprotective effects of exercise training systemically mediated? Prog. Cardiovasc. Dis. 2019, 62, 94–101. [Google Scholar] [CrossRef] [PubMed]
- de Castro Abrantes, H.; Briquet, M.; Schmuziger, C.; Restivo, L.; Puyal, J.; Rosenberg, N.; Rocher, A.B.; Offermanns, S.; Chatton, J.Y. The lactate receptor HCAR1 modulates neuronal network activity through the activation of Gα and Gβ ɣ subunits. J. Neurosci. 2019, 39, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Extracellular Vesicle-Associated RNA as a Carrier of Epigenetic Information. Genes 2017, 8, 240. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.; van Balkom, B.W.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.H.; Song, F.; Sun, Y. Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediat. Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Jahangiri, Z.; Gholamnezhad, Z.; Hosseini, M. Neuroprotective effects of exercise in rodent models of memory deficit and Alzheimer’s. Metab. Brain Dis. 2019, 34, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Trejo, J.L.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J. Neurosci. 2001, 21, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Saab, B.J.; Mansuy, I.M. Neuroepigenetics of memory formation and impairment: The role of microRNAs. Neuropharmacology 2014, 80, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Contrò, V.; Sacco, A.; Macchiarella, A.; Cieszczyk, P.; Proia, P. From Epigenetics to Anti-Doping Application: A New Tool of Detection. Hum. Mov. 2017, 18, 3–10. [Google Scholar] [CrossRef]
- Keifer, J.; Zheng, Z.; Ambigapathy, G. A MicroRNA-BDNF Negative Feedback Signaling Loop in Brain: Implications for Alzheimer’s Disease. Microrna 2015, 4, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, P.; Zhu, H.; Xu, Y.; Ma, C.; Dai, X.; Huang, L.; Liu, Y.; Zhang, L.; Qin, C. miR-34a, a microRNA up-regulated in a double transgenic of Alzheimer’s disease, inhibits bcl2 translation. Brain Res. Bull. 2009, 80, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Do, K.; Laing, B.T.; Landry, T.; Bunner, W.; Mersaud, N.; Matsubara, T.; Li, P.; Yuan, Y.; Lu, Q.; Huang, H. The effects of exercise on hypothalamic neurodegeneration of Alzheimer’s disease mouse model. PLoS ONE 2018, 13, e0190205. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- Hou, L.; Chen, W.; Liu, X.; Qiao, D.; Zhou, F.M. Exercise-induced neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 358. [Google Scholar] [CrossRef]
- Ibanez, P.; Bonnet, A.M.; Debarges, B.; Lohmann, E.; Tison, F.; Pollak, P.; Agid, Y.; Dürr, A.; Brice, A. Causal relation between α-synuclein gene duplication and familial Parkinson’s disease. Lancet 2004, 364, 1169–1171. [Google Scholar] [CrossRef]
- Nuytemans, K.; Theuns, J.; Cruts, M.; Van Broeckhoven, C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: A mutation update. Hum. Mutat. 2010, 31, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Lauzé, M.; Daneault, J.F.; Duval, C. The effects of physical activity in Parkinson’s disease: A Review. J. Parkinsons Dis. 2016, 6, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Bhalsing, K.S.; Abbas, M.M.; Tan, L.C.S. Role of Physical Activity in Parkinson’s Disease. Ann. Indian Acad. Neurol. 2018, 21, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.W.; Memon, A.A. Effects of exercise on non-motor symptoms in Parkinson’s disease. Clin. Ther. 2018, 40, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011, 77, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Tajiri, N.; Yasuhara, T.; Shingo, T.; Kondo, A.; Yuan, W.; Kadota, T.; Wang, F.; Baba, T.; Tayra, J.T.; Morimoto, T.; et al. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010, 1310, 200–207. [Google Scholar] [CrossRef]
- Collins, G.A.; Hill, L.E.; Chandramohan, Y.; Whitcomb, D.; Droste, S.K.; Reul, J.M. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate. PLoS ONE 2009, 4, e4330. [Google Scholar] [CrossRef]
- Cohen, A.D.; Tillerson, J.L.; Smith, A.D.; Schallert, T.; Zigmond, M.J. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: Possible role of GDNF. J. Neurochem. 2003, 85, 299–305. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Duzzioni, M.; Remor, A.P.; Tristão, F.S.; Matheus, F.C.; Raisman-Vozari, R.; Latini, A.; Prediger, R.D. Moderate-Intensity Physical Exercise Protects against Experimental 6-Hydroxydopamine-Induced Hemiparkinsonism Through Nrf2-Antioxidant Response Element Pathway. Neurochem. Res. 2016, 41, 64–72. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, T.W.; Park, S.S.; Han, J.H.; Shin, M.S.; Lim, B.V.; Kim, S.H.; Baek, S.S.; Cho, Y.S.; Kim, K.H. Treadmill Exercise Improves Motor Function by Suppressing Purkinje Cell Loss in Parkinson Disease Rats. Int. Neurourol. J. 2018, 22 (Suppl. 3), S147–S155. [Google Scholar] [CrossRef] [PubMed]
- Putcha, D.; Ross, R.S.; Cronin-Golomb, A.; Janes, A.C.; Stern, C.E. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. NeuroImage Clin. 2015, 7, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, C.; Kvint, S.; Frazzitta, G.; Bera, R.; Ferrazzoli, D.; Di Rocco, A.; Rebholz, H.; Friedman, E.; Pezzoli, G.; Quartarone, A.; et al. Intensive Rehabilitation Enhances Lymphocyte BDNF-TrkB Signaling in Patients with Parkinson’s Disease. Neurorehabil. Neural Repair. 2015, 30, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Crupi, D.; Liu, J.; Stucky, A.; Cruciata, G.; Di Rocco, A.; Friedman, E.; Quartarone, A.; Ghilardi, M.F. Repetitive Transcranial Magnetic Stimulation Enhances BDNF-TrkB Signaling in Both Brain and Lymphocyte. J. Neurosci. 2011, 31, 11044–11054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, N.A.; Wood, K.H.; Allendorfer, J.B.; Ford, M.P.; Bickel, C.S.; Marstrander, J.; Amara, A.W.; Anthony, T.; Bamman, M.M.; Skidmore, F.M. High-Intensity Exercise Acutely Increases Substantia Nigra and Prefrontal Brain Activity in Parkinson’s Disease. Med. Sci. Monit. 2017, 23, 6064–6071. [Google Scholar] [CrossRef]
- Kim-Ha, J.; Kim, Y.J. Age-related epigenetic regulation in the brain and its role in neuronal diseases. BMB Rep. 2016, 49, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, J.S.; Miller, A.C.; Hayes, T.; Shaw, E. Cognitive and behavioural changes in Huntington disease before diagnosis. Handb. Clin. Neurol. 2017, 144, 69–91. [Google Scholar] [CrossRef]
- Trembath, M.K.; Horton, Z.A.; Tippett, L.; Collins, V.R.; Churchyard, A.; Roxburgh, R. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov. Disord. 2010, 25, 1444–1450. [Google Scholar] [CrossRef]
- Mueller, S.M.; Petersen, J.A.; Jung, H.H. Exercise in Huntington’s disease: Current state and clinical significance. Tremor Other Hyperkinet. Mov. 2019, 9, 601. [Google Scholar] [CrossRef]
- Bohlen, S.; Ekwall, C.; Hellström, K.; Vesterlin, H.; Björnefur, M.; Wiklund, L.; Reilmann, R. Physical therapy in Huntington’s disease—Toward objective assessments? Eur. J. Neurol. 2013, 20, 389–393. [Google Scholar] [CrossRef]
- Cruickshank, T.M.; Thompson, J.A.; Domínguez, D.J.F.; Reyes, A.P.; Bynevelt, M.; Georgiou-Karistianis, N.; Barker, R.A.; Ziman, M.R. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: An exploratory study. Brain Behav. 2015, 5, e00312. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Hamana, K.; Kelson, M.; Dawes, H.; Collett, J.; Townson, J.; Roos, R.; van der Plas, A.A.; Reilmann, R.; Frich, J.C.; et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat. Disord. 2016, 31, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Downing, N.; Lourens, S.; Mills, J.; Kim, J.I.; Long, J.; Paulsen, J. Is There an Association of Physical Activity with Brain Volume, Behavior, and Day-to-day Functioning? A Cross Sectional Design in Prodromal and Early Huntington Disease. PLoS Curr. 2016, 17, 8. [Google Scholar] [CrossRef]
- Mueller, S.M.; Gehrig, S.M.; Petersen, J.A.; Frese, S.; Mihaylova, V.; Ligon-Auer, M.; Khmara, N.; Nuoffer, J.M.; Schaller, A.; Lundby, C.; et al. Effects of endurance training on skeletal muscle mitochondrial function in Huntington disease patients. Orph. J. Rare Dis. 2017, 12, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sølvsten, C.A.; de Paoli, F.; Christensen, J.H.; Nielsen, A.L. Voluntary physical exercise induces expression and epigenetic remodeling of VegfA in the rat hippocampus. Mol. Neurobiol. 2018, 55, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Contrò, V.; Schiera, G.; Macchiarella, A.; Sacco, A.; Lombardo, G.; Proia, P. Multiple sclerosis: Physical activity and well-being. Trends Sport Sci. 2017, 2, 53–58. [Google Scholar]
- Mähler, A.; Balogh, A.; Csizmadia, I.; Klug, L.; Kleinewietfeld, M.; Steiniger, J.; Šušnjar, U.; Müller, D.N.; Boschmann, M.; Paul, F. Metabolic, Mental and Immunological Effects of Normoxic and Hypoxic Training in Multiple Sclerosis Patients: A Pilot Study. Front. Immunol. 2018, 9, 2819. [Google Scholar] [CrossRef] [PubMed]
- Mulero, P.; Almansa, R.; Neri, M.J.; Bermejo-Martin, J.F.; Archanco, M.; Arenillas, J.F.; Téllez, N. Improvement of fatigue in multiple sclerosis by physical exercise is associated to modulation of systemic interferon response. J. Neuroimmunol. 2015, 280, 8–11. [Google Scholar] [CrossRef]
- Naghibzadeh, M.; Ranjbar, R.; Tabandeh, M.R.; Habibi, A. Effects of Two Training Programs on Transcriptional Levels of Neurotrophins and Glial Cells Population in Hippocampus of Experimental Multiple Sclerosis. Int. J. Sports Med. 2018, 39, 604–612. [Google Scholar] [CrossRef]
- Souza, P.S.; Goncalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.; Santos, A.R.; et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Houdebine, L.; Gallelli, C.A.; Rastelli, M.; Sampathkumar, N.K.; Grenier, J. Effect of physical exercise on brain and lipid metabolism in mouse models of multiple sclerosis. Chem. Phys. Lipids 2017, 207 Pt B, 127–134. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Arenas, E.; Denham, M.; Villaescusa, J.C. How to make a midbrain dopaminergic neuron. Development 2015, 142, 1918–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, J.F.; Zou, J.; Drouin-Ouellet, J.; Kim, K.Y.; Cicchetti, F.; Awatramani, R.B. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014, 9, 930–939. [Google Scholar] [CrossRef] [PubMed]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.W.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566–580. [Google Scholar] [CrossRef]
- Poulin, J.F.; Caronia, G.; Hofer, C.; Cui, Q.; Helm, B.; Ramakrishnan, C.; Chan, C.S.; Dombeck, D.A.; Deisseroth, K.; Awatramani, R. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 2018, 21, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Tiklová, K.; Björklund, Å.K.; Lahti, L.; Fiorenzano, A.; Nolbrant, S.; Gillberg, L.; Volakakis, N.; Yokota, C.; Hilscher, M.M.; Hauling, T.; et al. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef]
- Palmiter, R.D. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: Lessons from dopamine-deficient mice. Ann. N. Y. Acad. Sci. 2008, 1129, 35–46. [Google Scholar] [CrossRef]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shohamy, D.; Adcock, R.A. Dopamine and adaptive memory. Trends Cogn. Sci. 2010, 14, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.L.; Jones-Tabah, J.; O’Dowd, B.F.; George, S.R. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int. J. Neuropsychopharmacol. 2013, 16, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Pristerà, A.; Blomeley, C.; Lopes, E.; Threlfell, S.; Merlini, E.; Burdakov, D.; Cragg, S.; Guillemot, F.; Ang, S.L. Dopamine neuron-derived IGF-1 controls dopamine neuron firing, skill learning, and exploration. Proc. Natl. Acad. Sci. USA 2019, 116, 3817–3826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37 Pt B, 2243–2257. [Google Scholar] [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.J.; Fedynska, S.; Ghasem, P.R.; Wieman, T.; Clark, P.J.; Gray, N.; Loetz, E.; Campeau, S.; Fleshner, M.; Greenwood, B.N. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. Eur. J. Neurosci. 2016, 43, 1190–1202. [Google Scholar] [CrossRef]
- Zhu, X.; Ottenheimer, D.; DiLeone, R.J. Activity of D1/2 Receptor Expressing Neurons in the Nucleus Accumbens Regulates Running, Locomotion, and Food Intake. Front. Behav. Neurosci. 2016, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Nock, N.L.; Minnes, S.; Alberts, J.L. Neurobiology of substance use in adolescents and potential therapeutic effects of exercise for prevention and treatment of substance use disorders. Birth Defects Res. 2017, 109, 1711–1729. [Google Scholar] [CrossRef]
- Matta Mello, P.E.; Cevada, T.; Sobral Monteiro-Junior, R.; Teixeira, G.T.; da Cruz, R.E.; Lattari, E.; Blois, C.; Camaz Deslandes, A. Neuroscience of exercise: From neurobiology mechanisms to mental health. Neuropsychobiology 2013, 68, 1–14. [Google Scholar] [CrossRef]
- Crush, E.A.; Frith, E.; Loprinzi, P.D. Experimental effects of acute exercise duration and exercise recovery on mood state. J. Affect. Dis. 2018, 229, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.N. The role of dopamine in overcoming aversion with exercise. Brain Res. 2018, 1713, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Lightfoot, J.T. Does the difference between physically active and couch potato lie in the dopamine system? Int. J. Biol. Sci. 2010, 6, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Running from Disease: Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes. Front. Endocrinol. 2017, 8, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kami, K.; Tajima, F.; Senba, E. Activation of mesolimbic reward system via laterodorsal tegmental nucleus and hypothalamus in exercise-induced hypoalgesia. Sci. Rep. 2018, 8, 11540. [Google Scholar] [CrossRef] [PubMed]
- El Mestikawy, S.; Glowinski, J.; Hamon, M. Tyrosine hydroxylase activation in depolarized dopaminergic terminals--involvement of Ca2+-dependent phosphorylation. Nature 1983, 302, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, C.; Isobe, T.; Taoka, M.; Natsume, T.; Nomura, N.; Horigome, T.; Omata, S.; Ichinose, H.; Nagatsu, T.; Greene, L.A.; et al. Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry 1999, 38, 15673–15680. [Google Scholar] [CrossRef]
- Greenwood, B.N.; Foley, T.E.; Le, T.V.; Strong, P.V.; Loughridge, A.B.; Day, H.E.; Fleshner, M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011, 217, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Foley, T.E.; Fleshner, M. Neuroplasticity of dopamine circuits after exercise: Implications for central fatigue. Neuromol. Med. 2008, 10, 67–80. [Google Scholar] [CrossRef]
- Droste, S.K.; Schweizer, M.C.; Ulbricht, S.; Reul, J.M. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: Impact of concurrent treatment with the antidepressant drug tianeptine. J. Neuroendocrinol. 2006, 18, 915–925. [Google Scholar] [CrossRef]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front. Neuroendocrinol. 2017, 44, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Sutoo, D.; Akiyama, K. Regulation of brain function by exercise. Neurobiol. Dis. 2003, 13, 1–14. [Google Scholar] [CrossRef]
- Finberg, J.P.M. Inhibitors of MAO-B and COMT: Their effects on brain dopamine levels and uses in Parkinson’s disease. J. Neural. Transm. 2019, 126, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pine, D.S.; Ernst, M.; Gorodetsky, E.; Kasen, S.; Gordon, K.; Goldman, D.; Cohen, P. The MAOA gene predicts happiness in women. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.D.; Lorenzetti, M.S.; Lyle, S.M.; Fins, A.I.; Tartar, A.; Tartar, J.L. Catechol-O-methyltransferase Val158Met polymorphism associates with affect and cortisol levels in women. Brain Behav. 2018, 8, e00883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boekhoudt, L.; Omrani, A.; Luijendijk, M.C.; Wolterink-Donselaar, I.G.; Wijbrans, E.C.; van der Plasse, G.; Adan, R.A. Chemogenetic activation of dopamine neurons in the ventral tegmental area, but not substantia nigra, induces hyperactivity in rats. Eur. Neuropsychopharmacol. 2016, 26, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A. Endocannabinoids, exercise, pain, and a path to health with aging. Mol. Asp. Med. 2018, 64, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Brellenthin, A.G.; Crombie, K.M.; Hillard, C.J.; Koltyn, K.F. Endocannabinoid and Mood Responses to Exercise in Adults with Varying Activity Levels. Med. Sci. Sports Exerc. 2017, 49, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Abraham, W.; Aviv, W. Modulatory effects of cannabinoids on brain neurotransmission. Eur. J. Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- King-Himmelreich, T.S.; Möser, C.V.; Wolters, M.C.; Schmetzer, J.; Schreiber, Y.; Ferreirós, N.; Russe, O.Q.; Geisslinger, G.; Niederberger, E. AMPK contributes to aerobic exercise-induced antinociception downstream of endocannabinoids. Neuropharmacology 2017, 124, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13105–13108. [Google Scholar] [CrossRef] [PubMed]
- Sonnenblick, Y.; Taler, M.; Bachner, Y.G.; Strous, R.D. Exercise, Dehydroepiandrosterone (DHEA), and Mood Change: A Rationale for the “Runners High”? Isr. Med. Assoc. J. 2018, 20, 335–339. [Google Scholar] [PubMed]
- Prough, R.A.; Clark, B.J.; Klinge, C.M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 2016, 56, R139–R155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Kaneko, F.; Iwamoto, E.; Saitoh, S.; Yamada, T. Neuromuscular electrical stimulation increases serum brain-derived neurotrophic factor in humans. Exp. Brain Res. 2019, 237, 47–56. [Google Scholar] [CrossRef]
- Costa, M.T.S.; Vieira, L.P.; Barbosa, E.O.; Mendes Oliveira, L.; Maillot, P.; Ottero Vaghetti, C.A.; Giovani Carta, M.; Machado, S.; Gatica-Rojas, V.; Monteiro-Junior, R.S. Virtual Reality-Based Exercise with Exergames as Medicine in Different Contexts: A Short Review. Clin. Pract. Epidemiol. Ment. Health 2019, 15, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.; Evenhuis, H.M.; Hilgenkamp, T.I.M. Physical fitness of children and adolescent with moderate to severe intellectual disabilities. Disabil. Rehabil. 2019, 1–11. [Google Scholar] [CrossRef]
- Del Coso, J.; Moreno, V.; Gutiérrez-Hellín, J.; Baltazar-Martins, G.; Ruíz-Moreno, C.; Aguilar-Navarro, M.; Lara, B.; Lucía, A. ACTN3 R577X Genotype and Exercise Phenotypes in Recreational Marathon Runners. Genes 2019, 10, 413. [Google Scholar] [CrossRef]
- Kandel, E. The Disordered Mind: What Unusual Brains Tell Us about Ourselves; Little Brown Book Group, Ed.; Hachette: London, UK, 2018. [Google Scholar]

| Protocol/Aims [Ref] | Subjects/Studies Included | Methods of Analysis | Conclusions |
|---|---|---|---|
| Analysis based on a randomized controlled trial (Ballabeina Study: [65]) aimed at evidencing any relationship between aerobic fitness/motor skills and working memory and attention in pre-school children [59] | 245 ethnically diverse pre-school children (49% girl, mean age 5.2 years) were analysed at the beginning of the activity and 9 months later. | Physical tests: 1. Aerobic fitness, assessed according to the 20 m shuttle run [66], 2. Agility, assessed by an obstacle course, 3. Dynamic balance on a beam. In order to evaluate spatial memory and attention, each child was tested individually by focused tests. | Higher baseline aerobic fitness and motor skills were related to higher levels of working memory and attention. A further improvement of these latter abilities was noticed in the following 9 months. |
| The aim of the study was to ascertain whether very low-intensity exercise (i.e., walking), practiced during foreign-language (Polish) vocabulary encoding, improves subsequent recall, in comparison with encoding during physical rest [62] | 49 right-handed, monolingual, Germans, healthy subjects (aged 18–30 years). Criteria of exclusion: a history of psychiatric or neurological disorders, smoking, obesity, and any knowledge of Polish or other Slavic languages. | In the first session, participants learned 40 Polish words while walking on the motor-driven treadmill, at their previously determined preferred rate. In the second session, the participants learned a further group of 40 words, while sitting in a chair. Each session lasted 30 min. The order of sessions was different for different subjects, in a balanced way, and the experiments were repeated twice. | In both experiments, participants’ performance was better when they exercised during learning compared to learning when sedentary. Serum BDNF levels and salivary cortisol concentration were also measured: serum BDNF was unrelated to memory performance; on the other hand, a positive correlation between the salivary cortisol and the number of correctly recalled words was found. |
| The aim of the study was to clarify whether mnemonic discrimination is improved by an acute bout of moderate-intensity aerobic exercise [63] | 21 healthy young adults (mean age 20.5 ± 1.4 years, 10 females), without histories of neurological or psychiatric disorders. All participants had normal or corrected-to-normal vision, and normal colour vision. | In this study moderate intensity is defined as 40–59% of V̇O2 peak, as established by the American College of Sports Medicine (ACSM) [67]. The activity was performed by a recumbent ergometer. Mnemonic task: the participants were first shown 196 pictures of everyday objects and asked, for each of them, whether it was an indoor or an outdoor item. Then they were asked to identify by pressing a button, in the second group of 256 items, which were ‘previously seen’, ‘similar but not identical’ or ‘not previously seen’. | The lure discrimination index (LDI) for high-similarity items was higher after 10 min of moderate aerobic exercise than in resting controls, thus suggesting that a bout of acute aerobic exercise could improve pattern separation, that seems to rely on the dentate gyrus (DG) in humans. |
| The aim of the analysis was to search the literature, looking for evidence of chronic PA effects on mental health in children and adolescents [58]. | Review articles reporting chronic physical activity and at least one mental health outcome (i.e., depression, anxiety/stress, self-esteem and cognitive functioning) in children/adolescents. Reviews chosen: 4 papers on the evidence concerning PA and depression; 4 for anxiety; 3 for self-esteem; 7 for cognitive functions. | Analysis based on data collected from PubMed, SPORTDiscus, PsychINFO, Web of Science, Medline, Cochrane Library, and ISI Science Citation Index, by using search terms related to the variables of interest (e.g., sport, exercise, physical activity) and mental health outcome variables (e.g., depression, anxiety, self-esteem, cognitive functioning). | Associations between PA and mental health in young people (Tables 1–4 in Ref. [58]) is evident, but the effects are small-to-moderate, probably because of weakness of the research designs. Small but consistent association between sedentary time and poorer mental health is also evident. |
| The aim of this systematic review was to find out studies elucidating the relationship between aerobic PA and children’s cognition, academic achievement, and psychosocial function [60] | Studies analysed concerned interventions of aerobic PA in children younger than 19 years. Only randomized control trials that measured psychological, behavioural, cognitive, or academic outcomes were included. | The review was performed using MEDLINE, Cochrane, PsycINFO, SPORTDiscus, and EMBASE. Additional studies were identified through back-searching bibliographies. | Aerobic PA is positively associated with cognition, academic achievement, behaviour, and psychosocial functioning outcomes. More rigorous trials, however, required for deducing detailed relationships. |
| Systematic review and meta-analysis of studies concerning associations between PA/sedentary lifestyle and mental health. Meta-analyses were performed in randomized controlled trials (RCTs) and non-RCTs (i.e., quasi-experimental studies) [64] | Studies published from January 2013 to April 2018. Studies were included if they comprehended PA or sedentary behaviour data and at least one psychological ill-being (i.e., depression, anxiety, stress, etc.) or psychological well-being (i.e., self-esteem, optimism, happiness, etc.) outcome in pre-schoolers (2–5 years of age), children (6–11 years of age) or adolescents (12–18 years of age). | Analysis based on data collected through a systematic search of the PubMed and Web of Science databases by two independent researchers. A narrative synthesis of observational studies was conducted. | PA improves adolescents’ mental health, but additional studies are needed to confirm the effects of PA on children. Findings from observational studies, however, suggest that promoting PA and decreasing sedentary behaviour might have a protecting effect on mental health in both children and adolescents. |
| Protocol/Aims [Ref] | Subjects/Studies Included | Methods of Analysis | Conclusions |
|---|---|---|---|
| The aim of the study was to test the effects of two high-intensity exercise protocols, already known to improve cardiovascular health, to also affect BDNF levels [103] | Experiment 1: 8 men (average age: 28 years) Experiment 2: 21 men (average age: 27 years) Both experiments included: -high-intensity interval-training (HIT), at 90% of maximal work rate for 1 min, alternating with 1 min of rest; -continuous exercise (CON), at 70% of maximal work rate. Both protocols lasted 20 min. | Experiment 1: serum [BDNF] was measured at 30 min before starting the exercise, at 0, 6, 10, 14, and 18 min during the exercise, and at the end of the exercise (20 min). Experiment2: Serum BDNF was measured only at the beginning (0 min) and at the end (20 min) of the experiment. BDNF was evaluated by an enzyme-linked immunoassay (ELISA). | -Similar BDNF kinetics were observed in both protocols, with maximal BDNF level reached toward the end of training; -Both protocols (CON and HIT) significantly increased BDNF, with HIT more effective Shorter bouts of high-intensity exercise are slightly more effective than continuous high-intensity exercise for elevating serum BDNF. Moreover, 73% of the participants preferred the HIT protocol Thus, the authors suggest that the HIT is an effective and preferred intervention for elevating BDNF and potentially promoting brain health. |
| The aim of this analysis was to study the possible relationship between exercise intensity, memory, and BDNF [104] | 16 young subjects (average age: 23 years): 9 men and 7 women | 3 exercise sessions at different intensities relative to ventilator threshold (Vt) (VO2max, Vt − 20%, Vt + 20%). Each session lasted approximately 30 min. Following exercise, the Rey Auditory Verbal Learning Test (RAVLT) was performed to assess short-term memory, learning, and long-term memory recall. 24 h later, the participants completed the RAVLT recognition trial, to evaluate another measure of long-term memory. Blood was drawn before exercise, immediately post-exercise, and after the 30-min recall test. Serum BDNF was evaluated by ELISA. | Long-term memory as assessed after the 24-h delay differed as a function of exercise intensity: the largest benefits were observed with the maximal intensity exercise. BDNF significantly increased in response to exercise. However, no difference was noticed in relation to exercise intensity. Similarly, no significant association was found with memory. The authors suggest that “future research is warranted so that we can better understand how to use exercise to benefit cognitive performance”. |
| The aim of the study was to compare basal- and post-exercise- levels of circulating BDNF, in comparison with cognitive training and mindfulness practice [105] | 19 healthy subjects (age: 65–85 years) | Exercises: (1) physical aerobic exercise at a moderate level, using a Swedish version of the EA Sports Active 2™ program on a Microsoft Xbox360™ game console connected to a Microsoft Kinect™ accessory and an ordinary TV set; (2) cognitive training through a computerized working memory training program; (3) mindfulness practice through the use of the Mindfulness App (http://www.mindapps.se/themindfulnessapp/). Each program lasted 35 min. All the participants went through all the three training programs, in a random sequence. Serum BDNF was evaluated by ELISA. | Exercise caused a significant increase in BDNF levels. Moreover, in the same subject, a single bout of exercise had a significantly higher impact on serum BDNF levels than cognitive training and mindfulness practice. However, considerable variability of BDNF responses was found when comparing different subjects. |
| The aim of the study was to compare the effect of ‘open-skill’ with ‘closed-skill’ exercise (as defined in terms of predictability of context situations) on BDNF production [102] | 20 adult males: all subjects participated in both closed (running) and open (badminton) skill exercise sessions, in counterbalanced order on separate days. Exclusion criteria: - cardiovascular disease, diabetes, history of neurological problems, pre-existing injuries, smoking or intake of recreational drugs; hearing or vision problems. | Exercise sessions: −5 min of warm-up exercises, −30 min of running or badminton. Exercise intensity: 60% of the heart rate reserve level (HRR) During each session, venous blood samples were obtained immediately before and after exercise. Serum BDNF was evaluated by ELISA. Cognitive performance was also evaluated by a modified form of the task-switching paradigm, and controlled via the Neuroscan Stim software. | Badminton exercise resulted in significantly higher serum BDNF levels relative to running. This study provides interesting evidence in support of the benefits of open-skills exercise on BDNF production and executive function. |
| The aim of the study was to analyse the effect of aquarobic exercise on serum irisin and BDNF levels [106] | 26 elderly women: Control group: 12 subjects Exercise group: 14 | Exercise sessions: 16-week aquarobic exercise program, including two sessions a week. Each session lasted for 60 min: −10 min of warm-up, −40 min of exercise, −10 min of cool. Serum irisin and BDNF levels were evaluated (three times in the exercise group and two times in the control group) by ELISA. | Aquarobic exercises improve the serum irisin and BDNF levels. |
| The aim of this study was to evaluate the effect of long-term exercise on memory and biomarkers related to cognition and oxidative stress, in healthy middle-aged subjects [107] | 68 healthy men: Group 1: 21 young sedentary subjects (age: 17–25 years); Group 2: 16 young trained subjects (age: 18–25 years), Group 3: 25 middle-aged sedentary subjects (age: 47–67 years) Group 4: 24 middle-aged trained subjects (age: 46–68 years). Exclusion criteria: -history of severe disease, pain, cognitive deficiencies, head trauma. -use of neuroactive or psychoactive drugs or antioxidants. | Comparison of the BDNF levels in the four groups was performed by a two-way ANOVA. The effect of PA on cognitive abilities was evaluated by a combination of neuropsychological tests, among which: the Trail Making Test, Part A and Part B, the Wechsler Adult Intelligence Scale IV Digit Span Subtest32, the Stroop Interference Test31, the Computerized tests from Cambridge Neuropsychological Test Automated Battery (CANTAB software, Cambridge Cognition, UK), and the Free and Cued Selective Reminding Test (FCSRT)33 Serum BDNF levels were measured by ELISA. | The Free and Cued Immediate Recall tests showed significant improvements in memory in the middle-aged trained individuals when compared to the sedentary ones. A significantly lower resting level of serum BDNF (and plasma Cathepsin B) was observed in both trained groups. In particular, BDNF and CTSB levels were inversely correlated with weekly hours of exercise. |
| The aim of the analysis was to find out any exercise-dependent correlation between BDNF concentration and aerobic metabolism in healthy subjects [100] | Studies were included when they reported BDNF analysis before and after at least one session of exercise. Total studied included: 20 | Analysis based on papers collected from PubMed, Scopus, and Medline databases. | PA-induced BDNF increase is related to the amount of aerobic energy required in the exercise, in a dose-dependent manner. |
| Protocols: -Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) -Cochrane Handbook of Systematic Reviews of Interventions [108] | Inclusion criteria: studied conducted on adolescents trained with different exercise protocols, and including evaluations of pre- and post-intervention BDNF levels. | Data derived from PubMed, EMBASE, Scopus, ScienceDirect, Web of Science, SPORTDiscus, the Cochrane Central Register of Controlled Trials (CENTRAL), and CINAHL. | The results show that BDNF levels increase after interventions, regardless of whether the aerobic exercises were acute or chronic. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. https://doi.org/10.3390/genes10090720
Di Liegro CM, Schiera G, Proia P, Di Liegro I. Physical Activity and Brain Health. Genes. 2019; 10(9):720. https://doi.org/10.3390/genes10090720
Chicago/Turabian StyleDi Liegro, Carlo Maria, Gabriella Schiera, Patrizia Proia, and Italia Di Liegro. 2019. "Physical Activity and Brain Health" Genes 10, no. 9: 720. https://doi.org/10.3390/genes10090720
APA StyleDi Liegro, C. M., Schiera, G., Proia, P., & Di Liegro, I. (2019). Physical Activity and Brain Health. Genes, 10(9), 720. https://doi.org/10.3390/genes10090720