Role of GUCA1C in Primary Congenital Glaucoma and in the Retina: Functional Evaluation in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Human Tissue Samples
2.3. Animals
2.4. Next Generation Sequencing (NGS)
2.5. Quantitative Reverse Transcription PCR (qRT-PCR)
2.6. Western Blotting and Antibodies
2.7. Light Microscopy
2.8. Fluorescence Immunohistochemistry (FIHC)
2.9. CRISPR/Cas9 Gene Editing
2.10. Zebrafish DNA Extraction
2.11. Genotyping by High-Resolution Melting (HRM)
2.12. Characterization of the CRISPR/Cas9-Induced Mutation
2.13. Statistics
3. Results
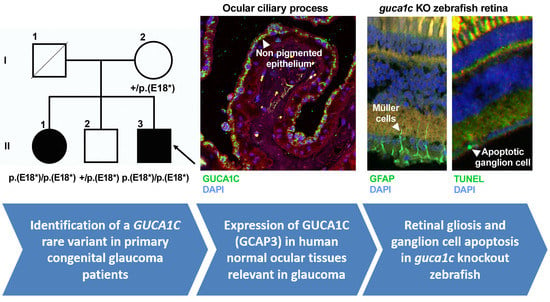
3.1. Identification of Rare GUCA1C Variants by WES
3.2. Clinical Features of Patients with the Nonsense GUCA1C Variant
3.3. Expression of GCAP3 in Adult Human Ocular Tissues
3.4. Functional Analysis of guca1c in Zebrafish
3.5. Phenotypic Characterization of guca1c KO Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chan, T.C.P.; Brookes, J.; Cavuoto, K.; Bitrian, E.; Grajewski, A.L. Primary congenital glaucoma and juvenile open-angle glaucoma. In Childhood Glaucoma; Weinreb, R.N., Grajewski, A.L., Papadopoulos, M., Grigg, J., Freedman, S., Eds.; Kluger Publications: Ámsterdam, The Netherlands, 2013; pp. 137–153. [Google Scholar]
- Francois, J. Congenital glaucoma and its inheritance. Ophthalmologica 1980, 181, 61–73. [Google Scholar] [CrossRef]
- Gencik, A. Epidemiology and genetics of primary congenital glaucoma in Slovakia. Description of a form of primary congenital glaucoma in gypsies with autosomal-recessive inheritance and complete penetrance. Dev. Ophthalmol. 1989, 16, 76–115. [Google Scholar]
- Elder, M.J. Congenital glaucoma in the West Bank and Gaza Strip. Br. J. Ophthalmol. 1993, 77, 413–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfarazi, M.; Stoilov, I. Molecular genetics of primary congenital glaucoma. Eye 2000, 14 Pt 3B, 422–428. [Google Scholar] [CrossRef]
- Weisschuh, N.; Wolf, C.; Wissinger, B.; Gramer, E. A clinical and molecular genetic study of German patients with primary congenital glaucoma. Am. J. Ophthalmol. 2009, 147, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Colomb, E.; Kaplan, J.; Garchon, H.J. Novel cytochrome P450 1B1 (CYP1B1) mutations in patients with primary congenital glaucoma in France. Hum. Mutat. 2003, 22, 496. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mollo, E.; Lopez-Garrido, M.-P.; Blanco-Marchite, C.; Garcia-Feijoo, J.; Peralta, J.; Belmonte-Martinez, J.; Ayuso, C.; Escribano, J. CYP1B1 mutations in Spanish patients with primary congenital glaucoma: Phenotypic and functional variability. Mol. Vis. 2009, 15, 417–431. [Google Scholar] [PubMed]
- López-Garrido, M.-P.; Medina-Trillo, C.; Morales-Fernandez, L.; Garcia-Feijoo, J.; Martínez-De-La-Casa, J.-M.; García-Antón, M.; Escribano, J. Null CYP1B1 genotypes in primary congenital and nondominant juvenile glaucoma. Ophthalmology 2013, 120, 716–723. [Google Scholar] [CrossRef]
- Ali, M.; McKibbin, M.; Booth, A.; Parry, D.A.; Jain, P.; Riazuddin, S.A.; Hejtmancik, J.F.; Khan, S.N.; Firasat, S.; Shires, M.; et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 2009, 84, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Narooie-Nejad, M.; Paylakhi, S.H.; Shojaee, S.; Fazlali, Z.; Rezaei, K.M.; Nilforushan, N.; Yazdani, S.; Babrzadeh, F.; Suri, F.; Ronaghi, M.; et al. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum. Mol. Genet. 2009, 18, 3969–3977. [Google Scholar] [CrossRef] [Green Version]
- Azmanov, D.N.; Dimitrova, S.; Florez, L.; Cherninkova, S.; Draganov, D.; Morar, B.; Saat, R.; Juan, M.; Arostegui, J.I.; Ganguly, S.; et al. LTBP2 and CYP1B1 mutations and associated ocular phenotypes in the Roma/Gypsy founder population. Eur. J. Hum. Genet. 2011, 19, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Souma, T.; Tompson, S.W.; Thomson, B.R.; Siggs, O.M.; Kizhatil, K.; Yamaguchi, S.; Feng, L.; Limviphuvadh, V.; Whisenhunt, K.N.; Maurer-Stroh, S.; et al. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J. Clin. Investig. 2016, 126, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Ferre-Fernández, J.J.; Aroca-Aguilar, J.D.; Medina-Trillo, C.; Bonet-Fernández, J.M.; Méndez-Hernández, C.D.; Morales-Fernández, L.; Corton, M.; Cabañero-Valera, M.J.; Gut, M.; Tonda, R.; et al. Whole-Exome Sequencing of Congenital Glaucoma Patients Reveals Hypermorphic Variants in GPATCH3, a New Gene Involved in Ocular and Craniofacial Development. Sci. Rep. 2017, 7, 46175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, K.; Reddy, A.B.; Mukhopadhyay, A.; Mandal, A.K.; Hasnain, S.E.; Ray, K.; Thomas, R.; Balasubramanian, D.; Chakrabarti, S. Myocilin gene implicated in primary congenital glaucoma. Clin. Genet. 2005, 67, 335–340. [Google Scholar] [CrossRef]
- Vincent, A.L.; Billingsley, G.; Buys, Y.; Levin, A.V.; Priston, M.; Trope, G.; Williams-Lyn, D.; Heon, E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am. J. Hum. Genet. 2002, 70, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Medina-Trillo, C.; Sánchez-Sánchez, F.; Aroca-Aguilar, J.D.; Ferre-Fernández, J.J.; Morales, L.; Méndez-Hernández, C.D.; Blanco-Kelly, F.; Ayuso, C.; García-Feijoo, J.; Escribano, J. Hypo- and hypermorphic FOXC1 mutations in dominant glaucoma: Transactivation and phenotypic variability. PLoS ONE 2015, 10, e0119272. [Google Scholar] [CrossRef]
- Medina-Trillo, C.; Aroca-Aguilar, J.-D.; Mendez-Hernandez, C.-D.; Morales, L.; Garcia-Anton, M.; Garcia-Feijoo, J.; Escribano, J. Rare FOXC1 variants in congenital glaucoma: Identification of translation regulatory sequences. Eur. J. Hum. Genet. 2016, 24, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Siggs, O.M.; Souzeau, E.; Taranath, D.A.; Dubowsky, A.; Chappell, A.; Zhou, T.; Javadiyan, S.; Nicholl, J.; Kearns, L.S.; Staffieri, S.E.; et al. Biallelic CPAMD8 Variants Are a Frequent Cause of Childhood and Juvenile Open-Angle Glaucoma. Ophthalmology 2020. [Google Scholar] [CrossRef]
- Bonet-Fernández, J.M.; Aroca-Aguilar, J.D.; Corton, M.; Ramírez, A.I.; Alexandre-Moreno, S.; García-Antón, M.T.; Salazar, J.J.; Ferre-Fernández, J.J.; Atienzar-Aroca, R.; Villaverde, C.; et al. CPAMD8 loss-of-function underlies non-dominant congenital glaucoma with variable anterior segment dysgenesis and abnormal extracellular matrix. Hum. Genet. 2020. [Google Scholar] [CrossRef]
- Ortego, J.; Escribano, J.; Becerra, S.; CocaPrados, M. Gene expression of the neurotrophic pigment epithelium-derived factor in the human ciliary epithelium—Synthesis and secretion into the aqueous humor. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2759–2767. [Google Scholar]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, OR, USA, 2000; p. 300. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Aroca-Aguilar, J.-D.; Martinez-Redondo, F.; Martin-Gil, A.; Pintor, J.; Coca-Prados, M.; Escribano, J. Bicarbonate-Dependent Secretion and Proteolytic Processing of Recombinant Myocilin. PLoS ONE 2013, 8, e54385. [Google Scholar] [CrossRef] [PubMed]
- Meeker, N.D.; Hutchinson, S.A.; Ho, L.; Trede, N.S. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques 2007, 43, 610, 612, 614. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.Z. Guanylate cyclase activators, cell volume changes and IOP reduction. Cell Physiol. Biochem. 2011, 28, 1145–1154. [Google Scholar] [CrossRef]
- Buys, E.S.; Potter, L.R.; Pasquale, L.R.; Ksander, B.R. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front. Mol. Neurosci. 2014, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, Y.; Yang, L.; Sokal, I.; Filipek, S.; Palczewski, K.; Baehr, W. Diversity of guanylate cyclase-activating proteins (GCAPs) in teleost fish: Characterization of three novel GCAPs (GCAP4, GCAP5, GCAP7) from zebrafish (Danio rerio) and prediction of eight GCAPs (GCAP1-8) in pufferfish (Fugu rubripes). J. Mol. Evol. 2004, 59, 204–217. [Google Scholar] [CrossRef] [Green Version]
- Rätscho, N.; Scholten, A.; Koch, K.W. Expression profiles of three novel sensory guanylate cyclases and guanylate cyclase-activating proteins in the zebrafish retina. Biochim. Biophys. Acta 2009, 1793, 1110–1114. [Google Scholar] [CrossRef] [Green Version]
- Fries, R.; Scholten, A.; Säftel, W.; Koch, K.W. Zebrafish guanylate cyclase type 3 signaling in cone photoreceptors. PLoS ONE 2013, 8, e69656. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, Y.; Li, N.; Sokal, I.; Sowa, M.E.; Lichtarge, O.; Wensel, T.G.; Saperstein, D.A.; Baehr, W.; Palczewski, K. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur. J. Neurosci. 2002, 15, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Haeseleer, F.; Sokal, I.; Li, N.; Pettenati, M.; Rao, N.; Bronson, D.; Wechter, R.; Baehr, W.; Palczewski, K. Molecular characterization of a third member of the guanylyl cyclase-activating protein subfamily. J. Biol. Chem. 1999, 274, 6526–6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuenca, N.; Lopez, S.; Howes, K.; Kolb, H. The localization of guanylyl cyclase-activating proteins in the mammalian retina. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1243–1250. [Google Scholar]
- Palczewski, K.; Sokal, I.; Baehr, W. Guanylate cyclase-activating proteins: Structure, function, and diversity. Biochem. Biophys. Res. Commun. 2004, 322, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Buys, E.S.; Ko, Y.C.; Alt, C.; Hayton, S.R.; Jones, A.; Tainsh, L.T.; Ren, R.; Giani, A.; Clerte’, M.; Abernathy, E.; et al. Soluble Guanylate Cyclase a1-Deficient Mice: A novel murine model for Primary Open Angle Glaucoma. Ann. Neurosci. 2013, 20, 65–66. [Google Scholar] [CrossRef] [Green Version]
- Buch, P.K.; Mihelec, M.; Cottrill, P.; Wilkie, S.E.; Pearson, R.A.; Duran, Y.; West, E.L.; Michaelides, M.; Ali, R.R.; Hunt, D.M. Dominant cone-rod dystrophy: A mouse model generated by gene targeting of the GCAP1/Guca1a gene. PLoS ONE 2011, 6, e18089. [Google Scholar] [CrossRef] [Green Version]
- Michaelides, M.; Wilkie, S.E.; Jenkins, S.; Holder, G.E.; Hunt, D.M.; Moore, A.T.; Webster, A.R. Mutation in the gene GUCA1A, encoding guanylate cyclase-activating protein 1, causes cone, cone-rod, and macular dystrophy. Ophthalmology 2005, 112, 1442–1447. [Google Scholar] [CrossRef]
- Nagy, E.; Maquat, L.E. A rule for termination-codon position within intron-containing genes: When nonsense affects RNA abundance. Trends Biochem. Sci. 1998, 23, 198–199. [Google Scholar] [CrossRef]
- Brogna, S.; Wen, J. Nonsense-mediated mRNA decay (NMD) mechanisms. Nat. Struct. Mol. Biol. 2009, 16, 107–113. [Google Scholar] [CrossRef]
- Mort, M.; Ivanov, D.; Cooper, D.N.; Chuzhanova, N.A. A meta-analysis of nonsense mutations causing human genetic disease. Hum. Mutat. 2008, 29, 1037–1047. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]








| Patient | II-3 |
|---|---|
| Age at diagnosis/age at last ophthalmic revision | Birth/48 years |
| IOP at diagnosis (mm Hg) (RE/LE) | NA |
| Last IOP (mm Hg) (RE/LE) | NA/17 |
| Last cup/disc ratio (RE/LE) | 0.9-1/0.9-1 |
| Number and type of glaucoma surgery (RE/LE) | 6 (3G, 3T)/5 (3G, 2T) |
| Number of antiglaucoma drugs (RE/LE) | 0/2, brimonidine tartrate and timolol |
| Visual acuity (RE/LE) | Amaurosis/Light perception |
| Lens | Cataract (B) |
| Central corneal thickness (m) (RE/LE) | 740/740 |
| Corneal morphology | MC (B), HS (B), L (B), BK and SE (RE) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Cámara, S.; Alexandre-Moreno, S.; Bonet-Fernández, J.-M.; Atienzar-Aroca, R.; Aroca-Aguilar, J.-D.; Ferre-Fernández, J.-J.; Méndez, C.-D.; Morales, L.; Fernández-Sánchez, L.; Cuenca, N.; et al. Role of GUCA1C in Primary Congenital Glaucoma and in the Retina: Functional Evaluation in Zebrafish. Genes 2020, 11, 550. https://doi.org/10.3390/genes11050550
Morales-Cámara S, Alexandre-Moreno S, Bonet-Fernández J-M, Atienzar-Aroca R, Aroca-Aguilar J-D, Ferre-Fernández J-J, Méndez C-D, Morales L, Fernández-Sánchez L, Cuenca N, et al. Role of GUCA1C in Primary Congenital Glaucoma and in the Retina: Functional Evaluation in Zebrafish. Genes. 2020; 11(5):550. https://doi.org/10.3390/genes11050550
Chicago/Turabian StyleMorales-Cámara, Samuel, Susana Alexandre-Moreno, Juan-Manuel Bonet-Fernández, Raquel Atienzar-Aroca, José-Daniel Aroca-Aguilar, Jesús-José Ferre-Fernández, Carmen-Dora Méndez, Laura Morales, Laura Fernández-Sánchez, Nicolas Cuenca, and et al. 2020. "Role of GUCA1C in Primary Congenital Glaucoma and in the Retina: Functional Evaluation in Zebrafish" Genes 11, no. 5: 550. https://doi.org/10.3390/genes11050550
APA StyleMorales-Cámara, S., Alexandre-Moreno, S., Bonet-Fernández, J. -M., Atienzar-Aroca, R., Aroca-Aguilar, J. -D., Ferre-Fernández, J. -J., Méndez, C. -D., Morales, L., Fernández-Sánchez, L., Cuenca, N., Coca-Prados, M., Martínez-de-la-Casa, J. -M., Garcia-Feijoo, J., & Escribano, J. (2020). Role of GUCA1C in Primary Congenital Glaucoma and in the Retina: Functional Evaluation in Zebrafish. Genes, 11(5), 550. https://doi.org/10.3390/genes11050550