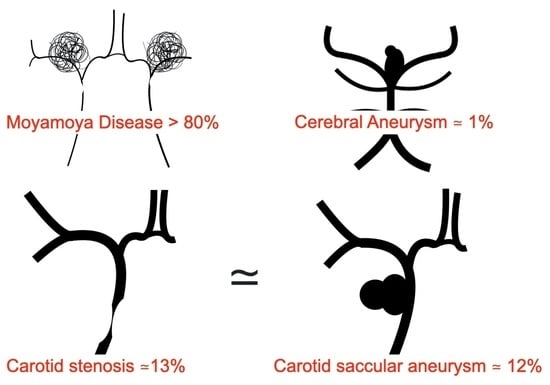
RNF213 c.14576G>A Is Associated with Intracranial Internal Carotid Artery Saccular Aneurysms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Information
2.2. Diagnosis
2.3. DNA Extraction and RNF213 Genotyping
2.4. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyawaki, S.; Imai, H.; Shimizu, M.; Yagi, S.; Ono, H.; Mukasa, A.; Nakatomi, H.; Shimizu, T.; Saito, N. Genetic variant RNF213 c.14576G>A in various phenotypes of intracranial major artery stenosis/occlusion. Stroke 2013, 44, 2894–2897. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Deng, J.; Dai, W.; Zhang, T.; Yan, J. Rare variants of RNF213 and moyamoya/non-moyamoya intracranial artery stenosis/occlusion disease risk: A meta-analysis and systematic review. Environ. Health Prev. Med. 2017, 22, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishisaka, E.; Watanabe, A.; Murai, Y.; Shirokane, K.; Matano, F.; Tsukiyama, A.; Baba, E.; Nakagawa, S.; Tamaki, T.; Mizunari, T.; et al. Role of RNF213 polymorphism in defining quasi-moyamoya disease and definitive moyamoya disease. Neurosurg. Focus 2021, 51, E2. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Tominaga, T.; Miyamoto, S.; Nagata, I.; Houkin, K.; Suzuki, N.; Takagi, Y. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. 2012, 52, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Bang, O.Y.; Chung, J.W.; Kim, D.H.; Won, H.H.; Yeon, J.Y.; Ki, C.S.; Shin, H.J.; Kim, J.S.; Hong, S.C.; Kim, D.K.; et al. Moyamoya Disease and Spectrums of RNF213 Vasculopathy. Transl. Stroke Res. 2020, 11, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Shinya, Y.; Miyawaki, S.; Imai, H.; Hongo, H.; Ono, H.; Takenobu, A.; Nakatomi, H.; Teraoka, A.; Saito, N. Genetic Analysis of Ring Finger Protein 213 (RNF213) c.14576G>A in Intracranial Atherosclerosis of the Anterior and Posterior Circulations. J. Stroke Cerebrovasc. Dis. 2017, 26, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Nie, F.; Li, Q.; Zhang, K.; Liu, M.; Yang, L.; Zhang, Q.; Liu, S.; Zeng, F.; et al. Association of Genetic Variants with Moyamoya Disease in 13,000 Individuals: A Meta-Analysis. Stroke 2020, 51, 1647–1655. [Google Scholar] [CrossRef]
- Zhou, S.; Ambalavanan, A.; Rochefort, D.; Xie, P.; Bourassa, C.V.; Hince, P.; Dionne-Laporte, A.; Spiegelman, D.; Gan-Or, Z.; Mirarchi, C.; et al. RNF213 Is Associated with Intracranial Aneurysms in the French-Canadian Population. Am. J. Hum. Genet. 2016, 99, 1072–1085. [Google Scholar] [CrossRef] [Green Version]
- Miyawaki, S.; Imai, H.; Takayanagi, S.; Mukasa, A.; Nakatomi, H.; Saito, N. Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke 2012, 43, 3371–3374. [Google Scholar] [CrossRef] [Green Version]
- The Natural Course of Unruptured Cerebral Aneurysms in a Japanese Cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhang, J. Neoplastic cerebral aneurysm from metastatic tumor: A systematic review of clinical and treatment characteristics. Clin. Neurol. Neurosurg. 2015, 128, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yoon, D.Y.; Kim, E.S.; Yun, E.J.; Jeon, H.J.; Lee, J.Y.; Cho, B.M. Geometric parameters on MRA source images to differentiate small Proximal Posterior communicating artery aneurysms from Infundibular dilation. J. Neuroimaging 2021, 31, 532–540. [Google Scholar] [CrossRef]
- Watanabe, A.; Karasugi, T.; Sawai, H.; Naing, B.T.; Ikegawa, S.; Orimo, H.; Shimada, T. Prevalence of c.1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasia in Japanese and effects of the mutation on heterozygous carriers. J. Hum. Genet. 2011, 56, 166–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashiro, R.; Fujimura, M.; Sakata, H.; Endo, H.; Tomata, Y.; Sato-Maeda, M.; Niizuma, K.; Tominaga, T. Genetic analysis of ring finger protein 213 (RNF213) c.14576G>A polymorphism in patients with vertebral artery dissection: A comparative study with moyamoya disease. Neurol. Res. 2019, 41, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Walkoff, L.; Brinjikji, W.; Rouchaud, A.; Caroff, J.; Kallmes, D.F. Comparing magnetic resonance angiography (MRA) and computed tomography angiography (CTA) with conventional angiography in the detection of distal territory cerebral mycotic and oncotic aneurysms. Interv. Neuroradiol. 2016, 22, 524–528. [Google Scholar] [CrossRef]
- Kamada, F.; Aoki, Y.; Narisawa, A.; Abe, Y.; Komatsuzaki, S.; Kikuchi, A.; Kanno, J.; Niihori, T.; Ono, M.; Ishii, N.; et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J. Hum. Genet. 2011, 56, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Morito, D.; Takashima, S.; Mineharu, Y.; Kobayashi, H.; Hitomi, T.; Hashikata, H.; Matsuura, N.; Yamazaki, S.; Toyoda, A.; et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS ONE 2011, 6, e22542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Kabata, R.; Kinoshita, H.; Morimoto, T.; Ono, K.; Takeda, M.; Choi, J.; Okuda, H.; Liu, W.; Harada, K.H.; et al. Rare variants in RNF213, a susceptibility gene for moyamoya disease, are found in patients with pulmonary hypertension and aggravate hypoxia-induced pulmonary hypertension in mice. Pulm. Circ. 2018, 8, 2045894018778155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, T.; Mineharu, Y.; Ono, K.; Nakatochi, M.; Ichihara, S.; Kabata, R.; Takagi, Y.; Cao, Y.; Zhao, L.; Kobayashi, H.; et al. Significant association of RNF213 p.R4810K, a moyamoya susceptibility variant, with coronary artery disease. PLoS ONE 2017, 12, e175649. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, M. Cardio-cephalic neural crest syndrome: A novel hypothesis of vascular neurocristopathy. Interv. Neuroradiol. 2017, 23, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Haldin, C.E.; LaBonne, C. SoxE factors as multifunctional neural crest regulatory factors. Int. J. Biochem. Cell Biol. 2010, 42, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Key, J.; Maletzko, A.; Kohli, A.; Gispert, S.; Torres-Odio, S.; Wittig, I.; Heidler, J.; Bárcena, C.; López-Otín, C.; Lei, Y.; et al. Loss of mitochondrial ClpP, Lonp1, and Tfam triggers transcriptional induction of Rnf213, a susceptibility factor for moyamoya disease. Neurogenetics 2020, 21, 187–203. [Google Scholar] [CrossRef]
| ICAN | ICS | p Value | |
|---|---|---|---|
| Sample size (N) | 49 | 22 | |
| RNF213 + | 6 | 3 | 0.573 |
| Male: Female | 11:38 | 13:9 | 0.0033 |
| Mean Age | 62.18 | 62.54 | 0.833 |
| Standard deviation | 13.82 | 11.18 | |
| Standard error | 1.975 | 2.384 | |
| Median | 65 | 65 | |
| Interquartile range | 20.5 | 19.5 | |
| Hypertension | 28 | 20 | 0.0038 |
| Diabetes mellitus | 5 | 9 | 0.0046 |
| Dyslipidemia | 20 | 14 | 0.0636 |
| Smoking | 24 | 14 | 0.1876 |
| ICAN and ICS | RNF + | RNF − | p Value |
|---|---|---|---|
| N | 9 | 62 | |
| Male: Female | 3:6 | 21:41 | 0.647 |
| Mean Age | 59.33 | 62.73 | 0.489 |
| Standard deviation | 14.87 | 12.77 | |
| Standard error | 4.96 | 1.62 | |
| Median | 65 | 65 | |
| Interquartile range | 29.5 | 19.5 | |
| Bilateral lesions | 2 | 12 | 0.755 |
| Hypertension | 7 | 41 | 0.389 |
| Diabetes mellitus | 1 | 13 | 0.431 |
| Dyslipidemia | 2 | 32 | 0.097 |
| Smoking | 4 | 34 | 0.409 |
| ICAN | RNF + | RNF − | p Value |
|---|---|---|---|
| Number | 6 | 43 | |
| Mean Age | 60.5 | 62.42 | 0.819 |
| Standard deviation | 17.24 | 13.51 | |
| Standard error | 7.04 | 2.06 | |
| Median | 69.5 | 65 | |
| Interquartile range | 33.5 | 20 | |
| Male | 1 | 10 | 0.592 |
| Proximal to Paraclinoid | 1 | 10 | 0.592 |
| Proximal to SHA | 1 | 19 | 0.204 |
| Bilateral ICAN | 0 | 6 | 0.436 |
| Bilateral CAN | 0 | 9 | 0.275 |
| Multiple ICAN | 2 | 10 | 0.46 |
| Multiple CAN | 2 | 14 | 0.649 |
| Family history of CAN | 1 | 2 | 0.33 |
| Ruptured CAN | 1 | 12 | 0.49 |
| Hypertension | 4 | 23 | 0.482 |
| Diabetes mellitus | 0 | 4 | 0.505 |
| Dyslipidemia | 1 | 18 | 0.204 |
| Smoking | 2 | 21 | 0.354 |
| ICS | RNF + | RNF − | p Value |
|---|---|---|---|
| Number | 3 | 19 | |
| Mean Age | 57 | 63.42 | 0.212 |
| Standard deviation | 11.36 | 11.21 | |
| Standard error | 6.56 | 2.57 | |
| Median | 62 | 66 | |
| Interquartile range | 21 | 21 | |
| Male | 2 | 11 | 0.642 |
| Bilateral stenosis | 2 | 3 | 0.117 |
| Hypertension | 3 | 17 | 0.74 |
| Diabetes mellitus | 1 | 8 | 0.642 |
| Dyslipidemia | 1 | 13 | 0.291 |
| Smoking | 2 | 12 | 0.764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murai, Y.; Ishisaka, E.; Watanabe, A.; Sekine, T.; Shirokane, K.; Matano, F.; Nakae, R.; Tamaki, T.; Koketsu, K.; Morita, A. RNF213 c.14576G>A Is Associated with Intracranial Internal Carotid Artery Saccular Aneurysms. Genes 2021, 12, 1468. https://doi.org/10.3390/genes12101468
Murai Y, Ishisaka E, Watanabe A, Sekine T, Shirokane K, Matano F, Nakae R, Tamaki T, Koketsu K, Morita A. RNF213 c.14576G>A Is Associated with Intracranial Internal Carotid Artery Saccular Aneurysms. Genes. 2021; 12(10):1468. https://doi.org/10.3390/genes12101468
Chicago/Turabian StyleMurai, Yasuo, Eitaro Ishisaka, Atsushi Watanabe, Tetsuro Sekine, Kazutaka Shirokane, Fumihiro Matano, Ryuta Nakae, Tomonori Tamaki, Kenta Koketsu, and Akio Morita. 2021. "RNF213 c.14576G>A Is Associated with Intracranial Internal Carotid Artery Saccular Aneurysms" Genes 12, no. 10: 1468. https://doi.org/10.3390/genes12101468
APA StyleMurai, Y., Ishisaka, E., Watanabe, A., Sekine, T., Shirokane, K., Matano, F., Nakae, R., Tamaki, T., Koketsu, K., & Morita, A. (2021). RNF213 c.14576G>A Is Associated with Intracranial Internal Carotid Artery Saccular Aneurysms. Genes, 12(10), 1468. https://doi.org/10.3390/genes12101468