Cancer Stem Cell Biomarkers Predictive of Radiotherapy Response in Rectal Cancer: A Systematic Review
Abstract
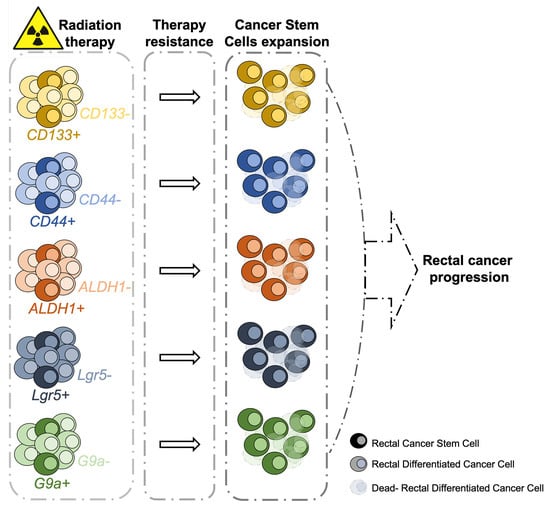
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Selection and Exclusion Criteria
2.3. Search Strategy
2.4. Quality Assessment and Risk of Bias
3. Results
3.1. Selection, Bias and Quality of Articles
3.2. Biomarkers Predictive of Radiotherapy Response in CSCs Isolated from RC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rodel, C.; Cervantes, A.; Arnold, D.; Committee, E.G. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv263. [Google Scholar] [CrossRef]
- Capelli, G.; De Simone, I.; Spolverato, G.; Cinquini, M.; Moschetti, I.; Lonardi, S.; Masi, G.; Carlomagno, C.; Corsi, D.; Luppi, G.; et al. Non-Operative Management Versus Total Mesorectal Excision for Locally Advanced Rectal Cancer with Clinical Complete Response After Neoadjuvant Chemoradiotherapy: A GRADE Approach by the Rectal Cancer Guidelines Writing Group of the Italian Association of Medical Oncology (AIOM). J. Gastrointest. Surg. 2020, 24, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [Green Version]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 806–815. [Google Scholar] [CrossRef]
- Couwenberg, A.M.; Burbach, J.P.M.; van Grevenstein, W.M.U.; Smits, A.B.; Consten, E.C.J.; Schiphorst, A.H.W.; Wijffels, N.A.T.; Heikens, J.T.; Intven, M.P.W.; Verkooijen, H.M. Effect of Neoadjuvant Therapy and Rectal Surgery on Health-related Quality of Life in Patients With Rectal Cancer During the First 2 Years After Diagnosis. Clin. Colorectal. Cancer 2018, 17, e499–e512. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Joo, J.; Kim, T.W.; Hong, Y.S.; Kim, J.E.; Hwang, I.G.; Kim, B.G.; Lee, K.W.; Kim, J.W.; Oh, H.S.; et al. A Randomized Phase 2 Trial of Consolidation Chemotherapy After Preoperative Chemoradiation Therapy Versus Chemoradiation Therapy Alone for Locally Advanced Rectal Cancer: KCSG CO 14-03. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 889–899. [Google Scholar] [CrossRef]
- Buckley, A.M.; Lynam-Lennon, N.; O’Neill, H.; O’Sullivan, J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 298–313. [Google Scholar] [CrossRef]
- Harada, Y.; Kazama, S.; Morikawa, T.; Emoto, S.; Murono, K.; Kaneko, M.; Sasaki, K.; Otani, K.; Nishikawa, T.; Tanaka, T.; et al. Prognostic impact of doublecortin-like kinase 1 expression in locally advanced rectal cancer treated with preoperative chemoradiotherapy. APMIS 2018, 126, 486–493. [Google Scholar] [CrossRef]
- Turdo, A.; Veschi, V.; Gaggianesi, M.; Chinnici, A.; Bianca, P.; Todaro, M.; Stassi, G. Meeting the Challenge of Targeting Cancer Stem Cells. Front. Cell Dev. Biol. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boman, B.M.; Wicha, M.S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26, 2795–2799. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.; Wei, X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine (Lond.) 2012, 7, 597–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peitzsch, C.; Kurth, I.; Ebert, N.; Dubrovska, A.; Baumann, M. Cancer stem cells in radiation response: Current views and future perspectives in radiation oncology. Int. J. Radiat. Biol. 2019, 95, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wei, J.W.; Tao, Y.L.; Ding, P.R.; Xia, Y.F.; Gao, Y.H.; Xiao, W.W. CCR6 Is a Predicting Biomarker of Radiosensitivity and Potential Target of Radiosensitization in Rectal Cancer. Cancer Res. Treat. 2018, 50, 1203–1213. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, S.M.; Kim, H.J.; Seo, A.N. Clinical influence of cancer stem cells on residual disease after preoperative chemoradiotherapy for rectal cancer. Tumour. Biol. 2016, 37, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Blazek, E.R.; Foutch, J.L.; Maki, G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1–5. [Google Scholar] [CrossRef]
- Woodward, W.A.; Chen, M.S.; Behbod, F.; Alfaro, M.P.; Buchholz, T.A.; Rosen, J.M. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl. Acad. Sci. USA 2007, 104, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Zhang, X.; Powell, K.; Li, L. Breast Cancer Stem Cells: Biomarkers, Identification and Isolation Methods, Regulating Mechanisms, Cellular Origin, and Beyond. Cancers 2020, 12, 3765. [Google Scholar] [CrossRef] [PubMed]
- Elkashty, O.A.; Abu Elghanam, G.; Su, X.; Liu, Y.; Chauvin, P.J.; Tran, S.D. Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 2020, 41, 458–466. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Alea, M.P.; Di Stefano, A.B.; Cammareri, P.; Vermeulen, L.; Iovino, F.; Tripodo, C.; Russo, A.; Gulotta, G.; Medema, J.P.; et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007, 1, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, N.; Ridgway, R.A.; van Es, J.H.; van de Wetering, M.; Begthel, H.; van den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Merlos-Suarez, A.; Barriga, F.M.; Jung, P.; Iglesias, M.; Cespedes, M.V.; Rossell, D.; Sevillano, M.; Hernando-Momblona, X.; da Silva-Diz, V.; Munoz, P.; et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 2011, 8, 511–524. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Ioue, Y.; Miki, C.; Kusunoki, M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Guo, W.H.; Meng, M.B.; Mo, X.M.; Lu, Y. Effects of heterochromatin in colorectal cancer stem cells on radiosensitivity. Chin. J. Cancer 2010, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Kawamoto, A.; Yasuda, H.; Morimoto, Y.; Fujikawa, H.; Inoue, Y.; et al. Immunohistochemical features of CD133 expression: Association with resistance to chemoradiotherapy in rectal cancer. Oncol. Rep. 2010, 24, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigusa, S.; Inoue, Y.; Tanaka, K.; Toiyama, Y.; Matsushita, K.; Kawamura, M.; Okugawa, Y.; Hiro, J.; Uchida, K.; Mohri, Y.; et al. Clinical significance of LGR5 and CD44 expression in locally advanced rectal cancer after preoperative chemoradiotherapy. Int. J. Oncol. 2012, 41, 1643–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.W.; Wang, J.Y.; Hung, W.C.; Peng, G.; Tsai, Y.L.; Chang, T.M.; Chai, C.Y.; Lin, C.H.; Pan, M.R. G9a governs colon cancer stem cell phenotype and chemoradioresistance through PP2A-RPA axis-mediated DNA damage response. Radiother. Oncol. 2017, 124, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Wu, C.; O’Rourke, K.P.; Szeglin, B.C.; Zheng, Y.; Sauve, C.G.; Adileh, M.; Wasserman, I.; Marco, M.R.; Kim, A.S.; et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat. Med. 2019, 25, 1607–1614. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, Y.N.; Zhang, K.; Zhao, Y.; Wu, Y.Y.; Li, G.; Cheng, H.Y.; Zhang, M.; Lai, F.; Wang, J.B.; et al. Polydatin Increases Radiosensitivity by Inducing Apoptosis of Stem Cells in Colorectal Cancer. Int. J. Biol. Sci. 2019, 15, 430–440. [Google Scholar] [CrossRef]
- Anuja, K.; Chowdhury, A.R.; Saha, A.; Roy, S.; Rath, A.K.; Kar, M.; Banerjee, B. Radiation-induced DNA damage response and resistance in colorectal cancer stem-like cells. Int. J. Radiat. Biol. 2019, 95, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Endo, H.; Kondo, J.; Onuma, K.; Ohue, M.; Inoue, M. Small subset of Wnt-activated cells is an initiator of regrowth in colorectal cancer organoids after irradiation. Cancer Sci. 2020, 111, 4429–4441. [Google Scholar] [CrossRef]
- Puglisi, C.; Giuffrida, R.; Borzi, G.; Di Mattia, P.; Costa, A.; Colarossi, C.; Deiana, E.; Picardo, M.C.; Colarossi, L.; Mare, M.; et al. Radiosensitivity of Cancer Stem Cells Has Potential Predictive Value for Individual Responses to Radiotherapy in Locally Advanced Rectal Cancer. Cancers 2020, 12, 3672. [Google Scholar] [CrossRef]
- Hewitt, H.B.; Wilson, C.W. The effect of tissue oxygen tension on the radiosensitivity of leukaemia cells irradiated in situ in the livers of leukaemic mice. Br. J. Cancer 1959, 13, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.P.; Milas, L. The proportion of stem cells in murine tumors. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 513–518. [Google Scholar] [CrossRef]
- Baumann, M.; Dubois, W.; Suit, H.D. Response of human squamous cell carcinoma xenografts of different sizes to irradiation: Relationship of clonogenic cells, cellular radiation sensitivity in vivo, and tumor rescuing units. Radiat. Res. 1990, 123, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 2008, 8, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Krause, M.; Baumann, M. Cancer stem cells at the crossroads of current cancer therapy failures—Radiation oncology perspective. Semin. Cancer Biol. 2010, 20, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Yaromina, A.; Eicheler, W.; Koch, U.; Baumann, M. Cancer stem cells: Targets and potential biomarkers for radiotherapy. Clin. Cancer Res. 2011, 17, 7224–7229. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Yoshioka, Y.; Isohashi, F.; Seo, Y.; Yoshida, K.; Yamazaki, H. Radiotherapy targeting cancer stem cells: Current views and future perspectives. Anticancer Res. 2013, 33, 747–754. [Google Scholar]
- Vermani, L.; Kumar, R.; Kannan, R.R.; Deka, M.K.; Talukdar, A.; Kumar, N.S. Expression pattern of ALDH1, E-cadherin, Vimentin and Twist in early and late onset sporadic colorectal cancer. Biomark. Med. 2020, 14, 1371–1382. [Google Scholar] [CrossRef]
- Stockton, J.D.; Tee, L.; Whalley, C.; James, J.; Dilworth, M.; Wheat, R.; Nieto, T.; Consortium, S.C.; Geh, I.; Barros-Silva, J.D.; et al. Complete response to neoadjuvant chemoradiotherapy in rectal cancer is associated with RAS/AKT mutations and high tumour mutational burden. Radiat. Oncol. 2021, 16, 129. [Google Scholar] [CrossRef]
- de Rosa, N.; Rodriguez-Bigas, M.A.; Chang, G.J.; Veerapong, J.; Borras, E.; Krishnan, S.; Bednarski, B.; Messick, C.A.; Skibber, J.M.; Feig, B.W.; et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J. Clin. Oncol. 2016, 34, 3039–3046. [Google Scholar] [CrossRef]
- Zhou, C.; Xiao, W.; Jiang, T.; Guo, Z.; Li, M.; Chang, H.; Wu, Y.; Chen, M.; Shi, M.; Xu, W.; et al. Targeting SGK1 enhances the efficacy of radiotherapy in locally advanced rectal cancer. Biomed. Pharmacother. 2020, 125, 109954. [Google Scholar] [CrossRef]
- Kim, J.H. Controversial issues in radiotherapy for rectal cancer: A systematic review. Radiat. Oncol. J. 2017, 35, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parashar, B.; Chen, W.C.; Herman, J.M.; Potters, L. Disease Site-Specific Guidelines for Curative Radiation Treatment During ‘Limited Surgery’ and ‘Hospital Avoidance’: A Radiation Oncology Perspective From the Epicenter of COVID-19 Pandemic. Cureus 2020, 12, e8190. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Lleonart, M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Bioessays 2016, 38 (Suppl. 1), S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.J.; Nozuhur, S.; RA, A.L.; Rodriguez-Enriquez, S.; Moreno-Sanchez, R. Repurposing drugs as pro-oxidant redox modifiers to eliminate cancer stem cells and improve the treatment of advanced stage cancers. Med. Res. Rev. 2019, 39, 2397–2426. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Islam, F.; Lam, A.K. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells 2020, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Butof, R.; Dubrovska, A.; Baumann, M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother. Oncol. 2013, 108, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Osman, A.A.; Takahashi, Y.; Lindemann, A.; Patel, A.A.; Zhao, M.; Takahashi, H.; Myers, J.N. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol. 2018, 87, 49–57. [Google Scholar] [CrossRef]
- Roeder, F.; Meldolesi, E.; Gerum, S.; Valentini, V.; Rodel, C. Recent advances in (chemo-)radiation therapy for rectal cancer: A comprehensive review. Radiat. Oncol. 2020, 15, 262. [Google Scholar] [CrossRef]
- Kuremsky, J.G.; Tepper, J.E.; McLeod, H.L. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 673–688. [Google Scholar] [CrossRef]



| No. | Question | Answer |
|---|---|---|
| Q1 | Is the study design well described? | Yes/No |
| Q2 | Is the study well written and the English language of sufficient quality? | Yes/No |
| Q3 | Is the experimental plan well organized? | Yes/No |
| Q4 | Are the results statistically significant? | Yes/No |
| Q5 | Are the positive/negative controls reported? | Yes/No |
| Q6 | Do the findings support the conclusions of the study? | Yes/No |
| Q7 | Are the human samples utilized ≥4? | Yes/No |
| Q8 | Is the study significant for the field ? | Yes/No |
| Q9 | Do the study cover the relevant literature in an unbiased manner? | Yes/No |
| Q10 | Is there any other source of bias in the study? | Yes/No |
| Study | Reference | Title of the Study | Patients and Samples | Biomarker | Biomarker Detection | Radiotherapy | Results |
|---|---|---|---|---|---|---|---|
| 1 | Saigusa S, Tanaka K, Toiyama Y, et al. 2009 [31] | Correlation of CD133, OCT4, and SOX2 in Rectal Cancer and Their Association with Distant Recurrence After chemoradiotherapy. | RC cells isolated from patients (TNM clinical stage II/III) pre- and postoperative CRT (n = 33). | CD133 OCT4 SOX2 | IHC Real-time PCR | Dose rate: Preoperative radiotherapy at 20–45 Gy. Postoperative radiotherapy with short-course radiation in 28 patients (20 Gy, five fractions over a week) or fractionated radiation in 5 patients (45 Gy, 18 fractions for 4 weeks). | Significant positive correlation between post-CRT levels of CD133, OCT4 and SOX2 and disease-free survival probability (p = 0.0285; p = 0.0114; p = 0.006). |
| 2 | Chen T, Zhang Y, Guo WH, et al. 2010 [32] | Effects of heterochromatin in colorectal cancer stem cells on radiosensitivity. | Human colorectal adenocarcinoma samples from patients (n = 16). | CD133 | Flow cytometry Immunofluorescence | Dose rate: 2 Gy/min (one side of the flank in nude mice was exposed to 10 Gy single dose of radiation, the other side without treatment served as control). | CSCs play a role in radiosensitivity in CRC, with a mechanism related to heterochromatin formation and histone methylation |
| 3 | Saigusa S, Tanaka K, Toiyama Y, et al. 2010 [33] | Immunohistochemical features of CD133 expression: Association with resistance to chemoradiotherapy in rectal cancer. | CSCs from RC patients (n = 50) and primary CRC patients (n = 40). | CD133 | IHC | Dose rate: 1, 2.5, and 5 Gy. | Correlation between CD133 expression and histopathological response to preoperative CRT. CD133 was also associated with resistance to CRT. |
| 4 | Saigusa S, Inoue Y, Tanaka K, et al. 2012 [34] | Clinical significance of LGR5 and CD44 expression in locally advanced rectal cancer after preoperative chemoradiotherapy. | RC specimens obtained from patients who underwent preoperative CRT (n = 52). | LGR5 CD44 | IHC | Dose rate: short-course (20 Gy in 4 fractions) or long-course (45 Gy in 25 fractions) radiotherapy. | Gene expression levels of LGR5 in cancer cells and CD44 in cancer stroma were significantly correlated with disease recurrence. High expression levels of stromal CD44 was an independent prognostic factor of recurrence and overall survival of RC patients after preoperative CRT. |
| 5 | Yoon G, Kim SM, Kim HJ, et al. 2016 [17] | Clinical influence of cancer stem cells on residual disease after preoperative chemoradiotherapy for rectal cancer. | Surgical specimens from patients with residual RC after CRT (n = 145). | ALDH1 CD44 | IHC | Dose rate: long-course radiation, 45 or 50 Gy in 25 fractions of 1.8 or 2 Gy administered to the whole pelvis five times per week for 5 weeks. | ALDH1 and CD44 positivity was related to lower TRG (p = 0.009; p = 0.003). ALDH1 positivity was associated short RFS and RCSS (p = 0.005 and 0.043 vs. p = 0.725 and 0.280, respectively). ALDH1 positivity was an independent prognostic factor for inferior RFS but not RCSS ((p = 0.039 vs. p = 0.571 [HR, 2.997; 95% CI, 1.059–8.478]). |
| 6 | Luo CW, Wang JY, Hung WC, et al. 2017 [35] | G9a governs colon cancer stem cell phenotype and chemoradioresistance through PP2A-RPA axis-mediated DNA damage response. | Primary tumors from patients who received preoperative CRT (n = 39) for RC and colorectal cancer cell lines (n = 3). | G9a CD133 | IHC Real-time PCR Flow cytometry | Dose rate: 20–45 Gy pelvic RT | Significantly positive correlation between G9a and CD133 in locally advanced RC patients receiving preoperative CRT. Knockdown of G9a increased the radiosensitivity of cells and sensitised cells to DNA damage agents through PP2A-RPA axis. |
| 7 | Ganesh K, Wu C, O’Rourke KP, et al. 2019 [36] | A rectal cancer organoid platform to study individual responses to chemoradiation. | RC tumoroids (n = 65) from n = 41 patients (22 from treatment-naïve patients; 43 from patients undergoing first- or second-line therapy) and normal rectal organoids from normal adjacent tissue (n = 51) | CDX2, nuclear β-catenin, Alcian blue, MUC-2, CK20, E-cadherin | IHC Immunofluorescence | Dose rate: 250 kVp and 12 mA | RC tumoroids display varying sensitivity to ionizing radiation, which corresponds to clinical radiotherapy responses. |
| 8 | Chen Q, Zeng YN, Zhang K, et al. 2019 [37] | Polydatin Increases Radiosensitivity by Inducing Apoptosis of Stem Cells in colorectal cancer. | C57BL/6 mouse model of CRC induced with AOM/DSS; CT26 and HCT116 colon cancer cells (n= 2). | Lgr5 | Flow cytometry | Dose rate: 10 Gy, 2 Gy/min, once a week for a total of four times | IR plus polydatin inhibit the proliferation and promote apoptosis of Lgr5+ CR-CSCs through the BMP signalling pathway |
| 9 | AnujaK, Chowdhury AR, Saha A, et al.2019 [38] | Radiation induced DNA damage response and resistance in colorectal cancer stem-like cells. | HCT116 and HCT-15 cells and derived clonospheres (n = 2). | CD44 KLF4 β-catenin TRF2 RAP1 hTERT | Real-time PCR Immunofluorescence | Dose rate: 4.0 Gy/min ([0–8 Gy] of 6Mv energy X-rays) | CSCs endowed with high DNA repair capacity survive following radiation therapy |
| 10 | Endo H, Kondo J, Onuma K, et al. 2020 [39] | Small subset of Wnt-activated cells is an initiator of regrowth in colorectal cancer organoids after irradiation. | Cancer tissue originated spheroid derived from CRC specimens (n = 4). | CD44v9, Wnt target genes, Lgr5 | Immunohistochemistry Immunofluorescence RT-PCR Real-time PCR | Dose rate: 9 Gy | Radiosensitivity differed among CTOS lines and showed good correlation with in vivo radiation sensitivity. Pre-treating organoids with HDACi increased radiosensitivity. Wnt inhibitors increased organoid radiosensitivity. |
| 11 | Puglisi C, Giuffrida R, Borzì G, et al. 2020 [40] | Radiosensitivity of cancer stem cells has potential predictive value for individual responses to radiotherapy in locally advanced rectal cancer. | CSC lines (n = 4) from CRC biopsies; animal models, generated by CSC xenotransplantation. | CD44, CD133 | Flow cytometry | Dose rate: Fractioned 25 Gy dose administered daily (5 Gy/Day) CSCs and animal models were subjected to in vitro irradiation with the same clinical protocol used for LARC patients | In vitro CSC radiosensitivity correspond to radiosensitive tumour xenografts upon subcutaneous implantation. CSCs’ in vitro and in vivo sensitivity values correspond to patients’ responses to radiotherapy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mare, M.; Colarossi, L.; Veschi, V.; Turdo, A.; Giuffrida, D.; Memeo, L.; Stassi, G.; Colarossi, C. Cancer Stem Cell Biomarkers Predictive of Radiotherapy Response in Rectal Cancer: A Systematic Review. Genes 2021, 12, 1502. https://doi.org/10.3390/genes12101502
Mare M, Colarossi L, Veschi V, Turdo A, Giuffrida D, Memeo L, Stassi G, Colarossi C. Cancer Stem Cell Biomarkers Predictive of Radiotherapy Response in Rectal Cancer: A Systematic Review. Genes. 2021; 12(10):1502. https://doi.org/10.3390/genes12101502
Chicago/Turabian StyleMare, Marzia, Lorenzo Colarossi, Veronica Veschi, Alice Turdo, Dario Giuffrida, Lorenzo Memeo, Giorgio Stassi, and Cristina Colarossi. 2021. "Cancer Stem Cell Biomarkers Predictive of Radiotherapy Response in Rectal Cancer: A Systematic Review" Genes 12, no. 10: 1502. https://doi.org/10.3390/genes12101502
APA StyleMare, M., Colarossi, L., Veschi, V., Turdo, A., Giuffrida, D., Memeo, L., Stassi, G., & Colarossi, C. (2021). Cancer Stem Cell Biomarkers Predictive of Radiotherapy Response in Rectal Cancer: A Systematic Review. Genes, 12(10), 1502. https://doi.org/10.3390/genes12101502