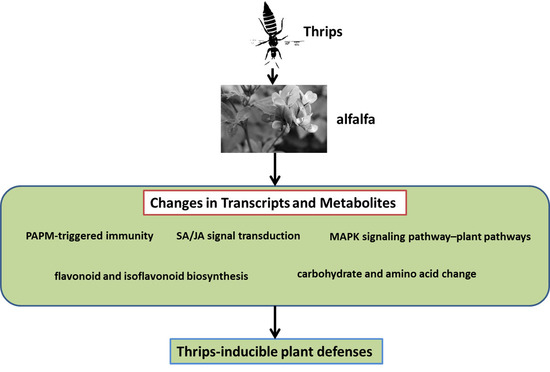
Combined Transcriptome and Metabolome Analysis of Alfalfa Response to Thrips Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Thrips Infection
2.2. RNA Extraction, cDNA Library Construction and RNA-Sequencing
2.3. De Novo Assembly, Annotation and Classification
2.4. Differentially Expressed Genes Analysis and Enrichment Analysis
2.5. Metabolome Analysis
2.6. Metabolomics Data Analysis
2.7. Combined Transcriptome and Metabolome Analyses
3. Results
3.1. Summary of Transcriptome and Metabolome Analysis
3.2. Differentially Expressed Genes and Differentially Accumulated Metabolites Analysis Related to Thrips Infection
3.3. Combined Transcriptome and Metabolome Analyses
4. Discussion
4.1. Primary Metabolites Changed Related to Thrips Infestation
4.2. Hormones Signaling Pathways Related to Thrips Infestation
4.3. Plant Immunity Signaling Pathways Related to Thrips Infestation
4.4. Plant Secondary Metabolites Pathway Related to Thrips Infestation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Temuer, B.; Zhao, X. A study on the alfalfa thrips. Acta Agrestia Sin. 1991, 1, 119–125. (In Chinese) [Google Scholar]
- Reitz, S.R. Biology and Ecology of the Western Flower Thrips (Thysanoptera: Thripidae): The Making of a Pest. Fla. Entomol. 2009, 92, 7–13. [Google Scholar] [CrossRef]
- Steenbergen, M.; Abd-El-Haliem, A.; Bleeker, P.; Dicke, M.; Escobar-Bravo, R.; Cheng, G.; Haring, M.A.; Kant, M.R.; Kappers, I.; Klinkhamer, P.G.L.; et al. Thrips advisor: Exploiting thrips-induced defences to combat pests on crops. J. Exp. Bot. 2018, 69, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Guide of Alfalfa Production and Management; Forestry: Beijing, China, 2003. [Google Scholar]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion Biology, Ecology and Management of Western Flower Thrips. Annu. Rev. Entomol. 2020, 51, 17–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Züst, T.; Agrawal, A.A. Trade-Offs between Plant Growth and Defense against Insect Herbivory: An Emerging Mechanistic Synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.Y.; Chu, C.C.; Henneberry, T.J. Frankliniella occidentalis colonization on okra- and normal-leaf cotton strains and cultivars. Southwest. Entomol. 2006, 31, 281–287. [Google Scholar]
- Khan, M.A.; Ali, A.; Aslam, M.; Tahir, Z.; Khan, M.M.; Nadeem, I. The role of morphological and chemical plant traits imparting resistance in bt cotton genotypes against thrips, Thrips tabaci (Lind.). Pak. J. Agric.-Cult. Sci. 2014, 51, 725–731. [Google Scholar]
- Miyazaki, J.; Stiller, W.N.; Wilson, L.J. Sources of plant resistance to thrips: A potential core component in cotton IPM. Entomol. Exp. Appl. 2017, 162, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Kariyat, R.R.; Balogh, C.M.; Moraski, R.P.; De Moraes, C.M.; Mescher, M.C.; Stephenson, A.G. Constitutive and herbivore-induced structural defenses are compromised by inbreeding in Solanum carolinense (Solanaceae). Am. J. Bot. 2013, 100, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Khorramdelazad, M.; Bar, I.; Whatmore, P.; Smetham, G.; Bhaaskaria, V.; Yang, Y.; Bai, S.H.; Mantri, N. Transcriptome profiling of lentil (Lens culinaris) through the first 24 hours of Ascochyta lentis infection reveals key defence response genes. BMC Genom. 2018, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.M.; Solorio Sanchez, F.J.; Ramirez, Y.; Aviles, L.; Mahdy, R.E.E.; Castillo Camaal, J.B. Tannins and mi-mosine in Leucaena genotypes and their relations to Leucaena resistance against Leucaena Psyllid and Onion thrips. Agrofor. Syst. 2017, 91, 1–8. [Google Scholar] [CrossRef]
- Hurej, M.; Kucharczyk, H.; Twardowski, J.P.; Kotecki, A. Thrips (Thysanoptera) associated with two genetically modified types of linseed (Linum usitatissimum L.). J. Plant Dis. Prot. 2017, 124, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Bertea, C.M.; Casacci, L.P.; Bonelli, S.; Zampollo, A.; Barbero, F. Chemical, Physiological and Molecular Responses of Host Plants to Lepidopteran Egg-Laying. Front. Plant Sci. 2020, 10, 1768. [Google Scholar] [CrossRef] [Green Version]
- Mellway, R.D.; Constabel, C.P. Metabolic engineering and potential functions of proanthocyanidins in poplar. Plant Signal. Behav. 2009, 4, 790–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirnezhad, M.; Romero-González, R.R.; Leiss, K.A.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L. Metabolomic Analysis of Host Plant Resistance to Thrips in Wild and Cultivated Tomatoes. Phytochem. Anal. 2010, 21, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Stoopen, G.; Wiegers, G.; Mao, J.; Wang, C.; Dicke, M.; Jongsma, M.A. Pyrethrins Protect Pyrethrum Leaves Against Attack by Western Flower Thrips, Frankliniella occidentalis. J. Chem. Ecol. 2012, 38, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-S.; Yang, C.-L.; Wang, S.-S.; Hu, G.-X. Changes of phenols and lignin contents in alfalfa leaf damaged by Odontothrips loti. J. Appl. Ecol. 2014, 25, 1688–1692. (In Chinese) [Google Scholar]
- Liu, X.; Klinkhamer, P.G.; Vrieling, K. The effect of structurally related metabolites on insect herbivores: A case study on pyrrolizidine alkaloids and western flower thrips. Phytochemistry 2017, 138, 93–103. [Google Scholar] [CrossRef]
- Tu, X.; Liu, Z.; Zhang, Z. Comparative transcriptomic analysis of resistant and susceptible alfalfa cultivars (Medicago sativa L.) after thrips infestation. BMC Genom. 2018, 19, 116. [Google Scholar] [CrossRef] [Green Version]
- Fracasso, A.; Trindade, L.M.; Amaducci, S. Drought stress tolerance strategies revealed by RNA-Seq in two sorghum genotypes with contrasting WUE. BMC Plant Biol. 2016, 16, 115. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Liu, W. De novo transcriptomic analysis during Lentinula edodes fruiting body growth. Gene 2018, 641, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, Y.; Li, B.; Tan, H.; Li, D.; Li, L.; Liu, X.; Han, J.; Meng, X. Comparative transcriptome analysis of genes involved in anthocyanin synthesis in blueberry. Plant Physiol. Bioch. 2018, 127, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Cock, P.J.A.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jozefczuk, S.; Klie, S.; Catchpole, G.; Szymanski, J.; Cuadros-Inostroza, A.; Steinhauser, D.; Selbig, J.; Willmitzer, L. Metabolomic and transcriptomic stress response of Escherichia coli. Mol. Syst. Biol. 2010, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.L.; Quisenberry, S.S. Global Plant Genetic Resources for Insect-Resistant Crops; CRC Press: Boca Raton, NY, USA, 1999. [Google Scholar]
- Kaur, B.; Kuraparthy, V.; Bacheler, J.; Fang, H.; Bowman, D.T. Screening Germplasm and Quantification of Com-ponents Contributing to Thrips Resistance in Cotton. J. Econ. Entomol. 2018, 111, 2426–2434. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.K.; Senthil Kumar, C.M.; Devasahayam, S.; D’Silva, S.; Kumar, R.S.; Biju, C.N.; Praveena, R.; Ankegowda, S.K.J. Plant morphological traits associated with field resistance to cardamom thrips (Sciothrips cardamomi) in cardamom (Elettaria cardamomum). Ann. Appl. Biol. 2020, 177, 143–151. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Klinkhamer, P.G.L.; Leiss, K.A. Induction of Jasmonic Acid-Associated Defenses by Thrips Alters Host Suitability for Conspecifics and Correlates with Increased Trichome Densities in Tomato. Plant Cell Physiol. 2017, 58, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-K.; Cao, L.-J.; Song, W.; Shi, P.; Gao, Y.-F.; Gong, Y.-J.; Chen, J.-C.; Hoffmann, A.A. Chromosome-level assembly of the melon thrips genome yields insights into evolution of a sap-sucking lifestyle and pesticide resistance. Mol. Ecol. Resour. 2020, 20, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Abney, M.R.; Lai, P.-C.; Culbreath, A.K.; Tallury, S.; Leal-Bertioli, S.C.M. Resistance to Thrips in Peanut and Implications for Management of Thrips and Thrips-Transmitted Orthotospoviruses in Peanut. Front. Plant Sci. 2018, 9, 1604. [Google Scholar] [CrossRef]
- Zhou, S.; Lou, Y.-R.; Tzin, V.; Jander, G. Alteration of Plant Primary Metabolism in Response to Insect Herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidallha, E.H.M.A.; Li, Y.; Li, H.; Chen, Y.; Tambel, L.I.M.; Hu, D.; Zhang, X.; Chen, D. Amino acid composition and level affect Bt protein concentration in Bt cotton. Plant Growth Regul. 2017, 82, 439–446. [Google Scholar] [CrossRef]
- Tsumuki, H.K.K.; Shiraga, T.; Kawada, K. Characteristics of barley resistance to cereal aphids. Nutritional differences between barley strains. Nogaku Kenkyu 1987, 61, 149–159. [Google Scholar] [CrossRef]
- Fan, J.; Crooks, C.; Creissen, G.; Hill, L.; Fairhurst, S.; Doerner, P.; Lamb, C. Pseudomonas sax Genes Overcome Aliphatic Isothiocyanate-Mediated Non-Host Resistance in Arabidopsis. Science 2011, 331, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Malik, U.; Barik, A. Effects of leaf epicuticular wax compounds from Solena amplexicaulis (Lam.) Gandhi on olfactory responses of a generalist insect herbivore. Allelopath. J. 2016, 37, 253–272. [Google Scholar]
- Reina-Pinto, J.J.; Yephremov, A. Surface lipids and plant defenses. Plant Physiol. Biochem. 2009, 47, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Zogli, P.; Pingault, L.; Grover, S.; Louis, J. Ento(o)mics: The intersection of “omic” approaches to decipher plant defense against sap-sucking insect pests. Curr. Opin. Plant Biol. 2020, 56, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Botha, A.M.; Lacock, L.; Van Niekerk, C.; Matsioloko, M.T.; Du Preez, F.B.; Loots, S.; Venter, E.; Kunert, K.J. Is photosynthetic transcriptional regulation in Triticum aestivum L. cv. “TugelaDN” a contributing factor for tolerance to Diuraphis noxia (Homoptera: Aphididae)? Plant Cell Rep. 2006, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, A.; Heng-Moss, T.; Sarath, G.; Twigg, P.; Xia, Y.; Lu, G.; Mornhinweg, D. Gene expression profiling of tolerant barley in response to Diuraphis noxia (Hemiptera: Aphididae) feeding. Bull. Entomol. Res. 2009, 99, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Sarde, S.J.; Bouwmeester, K.; Venegas-Molina, J.; David, A.; Boland, W.; Dicke, M. Involvement of sweet pepper CaLOX2 in jasmonate-dependent induced defence against Western flower thrips. J. Integr. Plant Biol. 2019, 61, 1085–1098. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Selig, P.; Keough, S.; Nalam, V.J.; Nachappa, P. Jasmonate-dependent plant defenses mediate soybean thrips and soybean aphid performance on soybean. Arthropod-Plant Interact. 2016, 10, 273–282. [Google Scholar] [CrossRef]
- Shalileh, S.; Ogada, P.A.; Moualeu, D.P.; Poehling, H.-M. Manipulation of Frankliniella occidentalis (Thysanoptera: Thripidae) by Tomato Spotted Wilt Virus (Tospovirus) Via the Host Plant Nutrients to Enhance Its Transmission and Spread. Environ. Entomol. 2016, 45, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temuer, B.; Si, Q. Anti-thrips alfalfa form features andf anti-insect sex research. J. Inn. Mong. Agric. Univ. 2014, 35, 51–58. (In Chinese) [Google Scholar]
- Malik, N.a.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Goldar, X.; Villari, C.; Bonello, P.; Borg-Karlson, A.K.; Grivet, D.; Zas, R.; Sampedro, L. Inducibility of Plant Secondary Metabolites in the Stem Predicts Genetic Variation in Resistance Against a Key Insect Herbivore in Maritime Pine. Front. Plant Sci. 2018, 9, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Wu, S.; Xing, Z.; Gao, Y.; Cai, W.; Lei, Z. Abundances of thrips on plants in vegetative and flowering stages are related to plant volatiles. J. Appl. Entomol. 2020, 144, 732–742. [Google Scholar] [CrossRef]
- Wink, M. Plant Secondary Metabolites Modulate Insect Behavior-Steps Toward Addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Soltis, N.E.; Kliebenstein, D.J. Natural Variation of Plant Metabolism: Genetic Mechanisms, Interpretive Caveats, and Evolutionary and Mechanistic Insights. Plant Physiol. 2015, 169, 1456–1468. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.; Zhang, L.; Panigrahi, P.; Dholakia, B.B.; Dewangan, V.; Chavan, S.G.; Kunjir, S.M.; Wu, X. Fusarium oxysporum mediates systems metabolic reprogramming of chickpea roots as revealed by a combination of proteomics and metabolomics. Plant Biotechnol. J. 2016, 14, 1589–1603. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wu, Q.; He, W.; He, T.; Wu, Q.; Miao, Y. Combined De Novo Transcriptome and Metabolome Analysis of Common Bean Response to Fusarium oxysporum f. sp. phaseoli Infection. Int. J. Mol. Sci. 2019, 20, 6278. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, Q.; Tan, Y.; Shuang, S.; Dai, R.; Jiang, X.; Temuer, B. Combined Transcriptome and Metabolome Analysis of Alfalfa Response to Thrips Infection. Genes 2021, 12, 1967. https://doi.org/10.3390/genes12121967
Zhang Z, Chen Q, Tan Y, Shuang S, Dai R, Jiang X, Temuer B. Combined Transcriptome and Metabolome Analysis of Alfalfa Response to Thrips Infection. Genes. 2021; 12(12):1967. https://doi.org/10.3390/genes12121967
Chicago/Turabian StyleZhang, Zhiqiang, Qi Chen, Yao Tan, Shuang Shuang, Rui Dai, Xiaohong Jiang, and Buhe Temuer. 2021. "Combined Transcriptome and Metabolome Analysis of Alfalfa Response to Thrips Infection" Genes 12, no. 12: 1967. https://doi.org/10.3390/genes12121967
APA StyleZhang, Z., Chen, Q., Tan, Y., Shuang, S., Dai, R., Jiang, X., & Temuer, B. (2021). Combined Transcriptome and Metabolome Analysis of Alfalfa Response to Thrips Infection. Genes, 12(12), 1967. https://doi.org/10.3390/genes12121967