The World of Oral Cancer and Its Risk Factors Viewed from the Aspect of MicroRNA Expression Patterns
Abstract
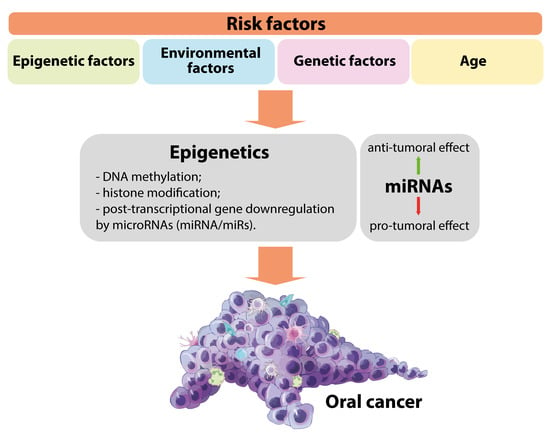
:1. Introduction
2. Major Risk Factors for Oral Cancer
2.1. Epigenetic Alteration Associated with Risk Factors
2.1.1. Tobacco Consumption
2.1.2. Betel Quid/Nut Chewing
2.1.3. Alcohol Consumption
2.1.4. Diet and Nutrition
2.1.5. Mouthwash
2.2. Environmental Factors
2.2.1. Viral Infections
2.2.2. Fungal Infections
2.2.3. Bacterial Infections
2.2.4. Occupational Risks
2.2.5. Poor Oral Health
2.3. Genetic Factors
2.4. Age
3. Oxidative Stress and Chronic Inflammation Associated with Risk Factors in Oral Cancer
4. The Functions of miRNAs Associated with Risk Factors in Oral Cancer
4.1. miRNAs Altered by Epigenetic Risk Factors
4.2. miRNAs Altered by Environmental Factors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trotta, B.M.; Pease, C.S.; Rasamny, J.J.; Raghavan, P.; Mukherjee, S. Oral cavity and oropharyngeal squamous cell cancer: Key imaging findings for staging and treatment planning. Radiographics 2011, 31, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Tseng, Y.K.; You, J.J.; Kang, B.H.; Wang, T.H.; Yang, C.M.; Chen, H.C.; Liou, H.H.; Liu, P.F.; Ger, L.P.; et al. Next-generation Sequencing for microRNA Profiling: MicroRNA-21-3p Promotes Oral Cancer Metastasis. Anticancer. Res. 2017, 37, 1059–1066. [Google Scholar] [PubMed] [Green Version]
- Chang, T.S.; Chang, C.M.; Ho, H.C.; Su, Y.C.; Chen, L.F.; Chou, P.; Lee, C.C. Impact of young age on the prognosis for oral cancer: A population-based study in Taiwan. PLoS ONE 2013, 8, e75855. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Waal, I. Are we able to reduce the mortality and morbidity of oral cancer; some considerations. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e33–e37. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.K.; Murphy, C.; Smith, A.B.; Kanatas, A.N.; Mitchell, D.A. Survival after surgery for oral cancer: A 30-year experience. Br. J. Oral Maxillofac. Surg. 2017, 55, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Kung, P.T.; Wang, S.T.; Huang, K.H.; Liu, S.A. Beneficial impact of multidisciplinary team management on the survival in different stages of oral cavity cancer patients: Results of a nationwide cohort study in Taiwan. Oral Oncol. 2015, 51, 105–111. [Google Scholar] [CrossRef]
- Wyss, A.; Hashibe, M.; Chuang, S.C.; Lee, Y.C.; Zhang, Z.F.; Yu, G.P.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 2013, 178, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; Dipalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I.A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health 2020, 17, 3168. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Hsiao, J.R.; Chang, C.C.; Lee, W.T.; Huang, C.C.; Ou, C.Y.; Tsai, S.T.; Chen, K.C.; Huang, J.S.; Wong, T.Y.; Lai, Y.H.; et al. The interplay between oral microbiome, lifestyle factors and genetic polymorphisms in the risk of oral squamous cell carcinoma. Carcinogenesis 2018, 39, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.A.; Weng, S.L.; Yang, S.F.; Chou, C.H.; Huang, W.C.; Tu, S.J.; Chang, T.H.; Huang, C.N.; Jong, Y.J.; Huang, H.D. A Three-MicroRNA Signature as a Potential Biomarker for the Early Detection of Oral Cancer. Int. J. Mol. Sci. 2018, 19, 758. [Google Scholar] [CrossRef] [Green Version]
- Hung, K.F.; Liu, C.J.; Chiu, P.C.; Lin, J.S.; Chang, K.W.; Shih, W.Y.; Kao, S.Y.; Tu, H.F. MicroRNA-31 upregulation predicts increased risk of progression of oral potentially malignant disorder. Oral Oncol. 2016, 53, 42–47. [Google Scholar] [CrossRef]
- Prete, R.D.; Ronga, L.; Addati, G.; Magrone, R.; Abbasciano, A.; Carlo, D.D.; Santacroce, L. A Retrospective Study about the Impact of Switching from Nested PCR to Multiplex Real-Time PCR on the Distribution of the Human Papillomavirus (HPV) Genotypes. Medicina 2019, 55, 418. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—Systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Tatullo, M.; Gentile, S.; Paduano, F.; Santacroce, L.; Marrelli, M. Crosstalk between oral and general health status in e-smokers. Medicine 2016, 95, e5589. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Cojocneanu, R.; Magdo, L.; Onaciu, A.; Ciocan, C.; Mehterov, N.; Dudea, D.; Buduru, S.; Berindan-Neagoe, I. Differential Effect of Smoking on Gene Expression in Head and Neck Cancer Patients. Int J. Environ. Res. Public Health 2018, 15, 1558. [Google Scholar] [CrossRef] [Green Version]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Zandberg, D.P.; Liu, S.; Goloubeva, O.; Ord, R.; Strome, S.E.; Suntharalingam, M.; Taylor, R.; Morales, R.E.; Wolf, J.S.; Zimrin, A.; et al. Oropharyngeal cancer as a driver of racial outcome disparities in squamous cell carcinoma of the head and neck: 10-year experience at the University of Maryland Greenebaum Cancer Center. Head Neck 2016, 38, 564–572. [Google Scholar] [CrossRef]
- Chang, E.T.; Liu, Z.; Hildesheim, A.; Liu, Q.; Cai, Y.; Zhang, Z.; Chen, G.; Xie, S.H.; Cao, S.M.; Shao, J.Y.; et al. Active and Passive Smoking and Risk of Nasopharyngeal Carcinoma: A Population-Based Case-Control Study in Southern China. Am. J. Epidemiol. 2017, 185, 1272–1280. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, D.C.; Wilson, L.F. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016, 44, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Gomez, L.; Giuliano, A.R.; Fulp, W.J.; Caudell, J.; Echevarria, M.; Sirak, B.; Abrahamsen, M.; Isaacs-Soriano, K.A.; Hernandez-Prera, J.C.; Wenig, B.M.; et al. Human Papillomavirus Genotype Detection in Oral Gargle Samples Among Men With Newly Diagnosed Oropharyngeal Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 460–466. [Google Scholar] [CrossRef]
- Shaw, R.J.; Liloglou, T.; Rogers, S.N.; Brown, J.S.; Vaughan, E.D.; Lowe, D.; Field, J.K.; Risk, J.M. Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: Quantitative evaluation using pyrosequencing. Br. J. Cancer 2006, 94, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, V.; Saranath, D. Concurrent hypermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol. 2004, 40, 145–153. [Google Scholar] [CrossRef]
- D’Souza, W.; Saranath, D. Clinical implications of epigenetic regulation in oral cancer. Oral Oncol. 2015, 51, 1061–1068. [Google Scholar] [CrossRef]
- Bai, Z.-T.; Bai, B.; Zhu, J.; Di, C.-X.; Li, X.; Zhou, W.-C. Epigenetic actions of environmental factors and promising drugs for cancer therapy. Oncol. Lett. 2018, 15, 2049–2056. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory Mechanisms of Epigenetic miRNA Relationships in Human Cancer and Potential as Therapeutic Targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef]
- Irimie, A.I.; Ciocan, C.; Gulei, D.; Mehterov, N.; Atanasov, A.G.; Dudea, D.; Berindan-Neagoe, I. Current Insights into Oral Cancer Epigenetics. Int. J. Mol. Sci. 2018, 19, 670. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Joosten, S.C.; Feng, Z.; de Ruijter, T.C.; Draht, M.X.; Melotte, V.; Smits, K.M.; Veeck, J.; Herman, J.G.; Van Neste, L.; et al. Analysis of DNA methylation in cancer: Location revisited. Nat. Rev. Clin. Oncol. 2018, 15, 459–466. [Google Scholar] [CrossRef]
- Loaeza-Loaeza, J.; Beltran, A.S.; Hernández-Sotelo, D. DNMTs and Impact of CpG Content, Transcription Factors, Consensus Motifs, lncRNAs, and Histone Marks on DNA Methylation. Genes 2020, 11, 1336. [Google Scholar] [CrossRef]
- Horii, T.; Hatada, I. Regulation of CpG methylation by Dnmt and Tet in pluripotent stem cells. J. Reprod. Dev. 2016, 62, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Saatci, C.; Caglayan, A.O.; Ozkul, Y.; Tahiri, S.; Turhan, A.B.; Dundar, M. Detection of p16 promotor hypermethylation in “Maras powder” and tobacco users. Cancer Epidemiol. 2009, 33, 47–50. [Google Scholar] [CrossRef]
- Takeshima, M.; Saitoh, M.; Kusano, K.; Nagayasu, H.; Kurashige, Y.; Malsantha, M.; Arakawa, T.; Takuma, T.; Chiba, I.; Kaku, T.; et al. High frequency of hypermethylation of p14, p15 and p16 in oral pre-cancerous lesions associated with betel-quid chewing in Sri Lanka. J. Oral Pathol. Med. 2008, 37, 475–479. [Google Scholar] [CrossRef]
- Liu, C.; Marioni, R.E.; Hedman Å, K.; Pfeiffer, L.; Tsai, P.C.; Reynolds, L.M.; Just, A.C.; Duan, Q.; Boer, C.G.; Tanaka, T.; et al. A DNA methylation biomarker of alcohol consumption. Mol. Psychiatry 2018, 23, 422–433. [Google Scholar] [CrossRef]
- Kato, K.; Hara, A.; Kuno, T.; Mori, H.; Yamashita, T.; Toida, M.; Shibata, T. Aberrant promoter hypermethylation of p16 and MGMT genes in oral squamous cell carcinomas and the surrounding normal mucosa. J. Cancer Res. Clin. Oncol. 2006, 132, 735–743. [Google Scholar] [CrossRef]
- Hema, K.N.; Smitha, T.; Sheethal, H.S.; Mirnalini, S.A. Epigenetics in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2017, 21, 252–259. [Google Scholar] [CrossRef]
- Goodchild, M.; Nargis, N.; Tursan d’Espaignet, E. Global economic cost of smoking-attributable diseases. Tob. Control. 2018, 27, 58–64. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, J.; Wang, J.; Huang, R. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tob. Induc. Dis. 2019, 17, 29. [Google Scholar] [CrossRef]
- Getz, K.R.; Rozek, L.S.; Peterson, L.A.; Bellile, E.L.; Taylor, J.M.G.; Wolf, G.T.; Mondul, A.M. Family history of cancer and head and neck cancer survival. Laryngoscope 2017, 127, 1816–1820. [Google Scholar] [CrossRef]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Maasland, D.H.; van den Brandt, P.A.; Kremer, B.; Goldbohm, R.A.; Schouten, L.J. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer 2014, 14, 187. [Google Scholar] [CrossRef] [Green Version]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and dental implants: A systematic review and meta-analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- Ramôa, C.P.; Eissenberg, T.; Sahingur, S.E. Increasing popularity of waterpipe tobacco smoking and electronic cigarette use: Implications for oral healthcare. J. Periodontal Res. 2017, 52, 813–823. [Google Scholar] [CrossRef]
- Pemberton, M.N. Oral cancer and tobacco: Developments in harm reduction. Br. Dent. J. 2018, 225, 822–826. [Google Scholar] [CrossRef]
- Maki, J. The incentives created by a harm reduction approach to smoking cessation: Snus and smoking in Sweden and Finland. Int. J. Drug Policy 2015, 26, 569–574. [Google Scholar] [CrossRef]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Lubin, J.H.; Purdue, M.; Kelsey, K.; Zhang, Z.F.; Winn, D.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; Smith, E.; et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: A pooled analysis of case-control studies. Am. J. Epidemiol. 2009, 170, 937–947. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K. Nicotine Delivery to the Aerosol of a Heat-Not-Burn Tobacco Product: Comparison With a Tobacco Cigarette and E-Cigarettes. Nicotine Tob. Res. 2018, 20, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, Y.; Jiang, X.; Zhang, H.; Zhu, F.; Hu, S.; Hou, H.; Hu, Q.; Pang, Y. Chemical Analysis and Simulated Pyrolysis of Tobacco Heating System 2.2 Compared to Conventional Cigarettes. Nicotine Tob. Res. 2019, 21, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Titz, B.; Sewer, A.; Lo Sasso, G.; Scotti, E.; Schlage, W.K.; Mathis, C.; Leroy, P.; Majeed, S.; Torres, L.O.; et al. Comparative systems toxicology analysis of cigarette smoke and aerosol from a candidate modified risk tobacco product in organotypic human gingival epithelial cultures: A 3-day repeated exposure study. Food Chem. Toxicol. 2017, 101, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Sewer, A.; Mathis, C.; Iskandar, A.R.; Kostadinova, R.; Schlage, W.K.; Leroy, P.; Majeed, S.; Guedj, E.; Trivedi, K.; et al. Systems Toxicology Assessment of the Biological Impact of a Candidate Modified Risk Tobacco Product on Human Organotypic Oral Epithelial Cultures. Chem. Res. Toxicol. 2016, 29, 1252–1269. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, F.; Sewer, A.; Scotti, E.; Titz, B.; Schlage, W.K.; Leroy, P.; Kondylis, A.; Vuillaume, G.; Iskandar, A.R.; Guedj, E.; et al. Assessment of the impact of aerosol from a potential modified risk tobacco product compared with cigarette smoke on human organotypic oral epithelial cultures under different exposure regimens. Food Chem. Toxicol. 2018, 115, 148–169. [Google Scholar] [CrossRef]
- Clarke, E.; Thompson, K.; Weaver, S.; Thompson, J.; O’Connell, G. Snus: A compelling harm reduction alternative to cigarettes. Harm Reduct. J. 2019, 16, 62. [Google Scholar] [CrossRef]
- Talukdar, F.R.; Ghosh, S.K.; Laskar, R.S.; Mondal, R. Epigenetic, Genetic and Environmental Interactions in Esophageal Squamous Cell Carcinoma from Northeast India. PLoS ONE 2013, 8, e60996. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.M.; Murphy, S.E.; Stepanov, I.; Wang, R.; Carmella, S.G.; Nelson, H.H.; Hatsukami, D.; Hecht, S.S. 2-Phenethyl Isothiocyanate, Glutathione S-transferase M1 and T1 Polymorphisms, and Detoxification of Volatile Organic Carcinogens and Toxicants in Tobacco Smoke. Cancer Prev. Res. 2016, 9, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Bhat, F.A.; Patil, S.; Routray, S.; Mohanty, N.; Nair, B.; Sidransky, D.; Ganesh, M.S.; Ray, J.G.; Gowda, H.; et al. Whole-Exome Sequencing Analysis of Oral Squamous Cell Carcinoma Delineated by Tobacco Usage Habits. Front. Oncol. 2021, 11, 660696. [Google Scholar] [CrossRef]
- Sun, Y.W.; Chen, K.M.; Imamura Kawasawa, Y.; Salzberg, A.C.; Cooper, T.K.; Caruso, C.; Aliaga, C.; Zhu, J.; Gowda, K.; Amin, S.; et al. Hypomethylated Fgf3 is a potential biomarker for early detection of oral cancer in mice treated with the tobacco carcinogen dibenzo[def,p]chrysene. PLoS ONE 2017, 12, e0186873. [Google Scholar] [CrossRef]
- Ghantous, Y.; Schussel, J.L.; Brait, M. Tobacco and alcohol-induced epigenetic changes in oral carcinoma. Curr. Opin. Oncol. 2018, 30, 152–158. [Google Scholar] [CrossRef]
- Chang, H.W.; Ling, G.S.; Wei, W.I.; Yuen, A.P. Smoking and drinking can induce p15 methylation in the upper aerodigestive tract of healthy individuals and patients with head and neck squamous cell carcinoma. Cancer 2004, 101, 125–132. [Google Scholar] [CrossRef]
- Breitling, L.P.; Yang, R.; Korn, B.; Burwinkel, B.; Brenner, H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am. J. Hum. Genet. 2011, 88, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Furniss, C.S.; Marsit, C.J.; Houseman, E.A.; Eddy, K.; Kelsey, K.T. Line region hypomethylation is associated with lifestyle and differs by human papillomavirus status in head and neck squamous cell carcinomas. Cancer Epidemiol. Biomark. Prev. 2008, 17, 966–971. [Google Scholar] [CrossRef] [Green Version]
- Supic, G.; Kozomara, R.; Jovic, N.; Zeljic, K.; Magic, Z. Prognostic significance of tumor-related genes hypermethylation detected in cancer-free surgical margins of oral squamous cell carcinomas. Oral Oncol. 2011, 47, 702–708. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, S.; Christianson, L.; Rehman, S.; Ekwunife, O.; Samkange-Zeeb, F. Smokeless Tobacco and Oral Potentially Malignant Disorders in South Asia: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2017, 20, 12–21. [Google Scholar] [CrossRef]
- Islam, S.; Muthumala, M.; Matsuoka, H.; Uehara, O.; Kuramitsu, Y.; Chiba, I.; Abiko, Y. How Each Component of Betel Quid Is Involved in Oral Carcinogenesis: Mutual Interactions and Synergistic Effects with Other Carcinogens—A Review Article. Curr. Oncol. Rep. 2019, 21, 53. [Google Scholar] [CrossRef]
- Sharan, R.N.; Mehrotra, R.; Choudhury, Y.; Asotra, K. Association of betel nut with carcinogenesis: Revisit with a clinical perspective. PLoS ONE 2012, 7, e42759. [Google Scholar] [CrossRef] [Green Version]
- Athukorala, I.A.; Tilakaratne, W.M.; Jayasinghe, R.D. Areca Nut Chewing: Initiation, Addiction, and Harmful Effects Emphasizing the Barriers and Importance of Cessation. J. Addict. 2021, 2021, 9967097. [Google Scholar] [CrossRef]
- Anand, R.; Dhingra, C.; Prasad, S.; Menon, I. Betel nut chewing and its deleterious effects on oral cavity. J. Cancer Res. Ther. 2014, 10, 499–505. [Google Scholar]
- Lai, Z.L.; Tsou, Y.A.; Fan, S.R.; Tsai, M.H.; Chen, H.L.; Chang, N.W.; Cheng, J.C.; Chen, C.M. Methylation-associated gene silencing of RARB in areca carcinogens induced mouse oral squamous cell carcinoma. BioMed Res. Int. 2014, 2014, 378358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.C.; Chan, C.P.; Chen, Y.J.; Hsien, H.C.; Chang, Y.C.; Yeung, S.Y.; Jeng, P.Y.; Cheng, R.H.; Hahn, L.J.; Jeng, J.H. Areca nut components stimulate ADAM17, IL-1α, PGE2 and 8-isoprostane production in oral keratinocyte: Role of reactive oxygen species, EGF and JAK signaling. Oncotarget 2016, 7, 16879–16894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.H.; Hsia, S.M.; Shih, Y.H.; Shieh, T.M. Association of Smoking, Alcohol Use, and Betel Quid Chewing with Epigenetic Aberrations in Cancers. Int. J. Mol. Sci. 2017, 18, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Marziliano, A.; Teckie, S.; Diefenbach, M.A. Alcohol-related head and neck cancer: Summary of the literature. Head Neck 2020, 42, 732–738. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2010, 5, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.P.; Siwakoti, B.; Sapkota, A.; Gautam, D.K.; Lee, Y.A.; Monroe, M.; Hashibe, M. Tobacco smoking, chewing habits, alcohol drinking and the risk of head and neck cancer in Nepal. Int. J. Cancer 2020, 147, 866–875. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Jiang, Y.; Guan, Y.; Wang, G.; Zhao, G. Smoking cessation mutually facilitates alcohol drinking cessation among tobacco and alcohol co-users: A cross-sectional study in a rural area of Shanghai, China. Tob. Induc. Dis. 2019, 17, 85. [Google Scholar]
- Kawakita, D.; Matsuo, K. Alcohol and head and neck cancer. Cancer Metastasis Rev. 2017, 36, 425–434. [Google Scholar] [CrossRef]
- Quertemont, E. Genetic polymorphism in ethanol metabolism: Acetaldehyde contribution to alcohol abuse and alcoholism. Mol. Psychiatry 2004, 9, 570–581. [Google Scholar] [CrossRef] [Green Version]
- Tang, K.; Li, Y.; Zhang, Z.; Gu, Y.; Xiong, Y.; Feng, G.; He, L.; Qin, S. The PstI/RsaI and DraI polymorphisms of CYP2E1 and head and neck cancer risk: A meta-analysis based on 21 case-control studies. BMC Cancer 2010, 10, 575. [Google Scholar] [CrossRef] [Green Version]
- Ramani, V.K.; Vinod, G.D.; Benny, N.; Naik, R. Characteristics of tobacco consumption among cancer patients at a tertiary cancer hospital in South India-A cross-sectional study. Tob. Use Insights 2021, 14, 1179173x211050395. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; Fernandez, L.; et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Silva, P.; Latruffe, N.; Gaetano, G. Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus. Molecules 2020, 25, 2569. [Google Scholar] [CrossRef]
- Theruvathu, J.A.; Jaruga, P.; Nath, R.G.; Dizdaroglu, M.; Brooks, P.J. Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 2005, 33, 3513–3520. [Google Scholar] [CrossRef] [Green Version]
- Yu, V.; Singh, P.; Rahimy, E.; Zheng, H.; Kuo, S.Z.; Kim, E.; Wang-Rodriguez, J.; Ongkeko, W.M. RNA-seq analysis identifies key long non-coding RNAs connected to the pathogenesis of alcohol-associated head and neck squamous cell carcinoma. Oncol. Lett. 2016, 12, 2846–2853. [Google Scholar] [CrossRef]
- Saad, M.A.; Kuo, S.Z.; Rahimy, E.; Zou, A.E.; Korrapati, A.; Rahimy, M.; Kim, E.; Zheng, H.; Yu, M.A.; Wang-Rodriguez, J.; et al. Alcohol-dysregulated miR-30a and miR-934 in head and neck squamous cell carcinoma. Mol. Cancer 2015, 14, 181. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef]
- Chuang, S.C.; Jenab, M.; Heck, J.E.; Bosetti, C.; Talamini, R.; Matsuo, K.; Castellsague, X.; Franceschi, S.; Herrero, R.; Winn, D.M.; et al. Diet and the risk of head and neck cancer: A pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012, 23, 69–88. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef]
- Nosrati, N.; Bakovic, M.; Paliyath, G. Molecular Mechanisms and Pathways as Targets for Cancer Prevention and Progression with Dietary Compounds. Int. J. Mol. Sci. 2017, 18, 2050. [Google Scholar] [CrossRef] [Green Version]
- Toporcov, T.N.; Tavares, G.E.; Rotundo, L.D.; Vaccarezza, G.F.; Biazevic, M.G.; Brasileiro, R.S.; de Carvalho, M.B.; Michaluart, P., Jr.; Kowalski, L.P.; Antunes, J.L. Do tobacco and alcohol modify protective effects of diet on oral carcinogenesis? Nutr. Cancer 2012, 64, 1182–1189. [Google Scholar] [CrossRef]
- Rossi, M.; Garavello, W.; Talamini, R.; Negri, E.; Bosetti, C.; Dal Maso, L.; Lagiou, P.; Tavani, A.; Polesel, J.; Barzan, L.; et al. Flavonoids and the risk of oral and pharyngeal cancer: A case-control study from Italy. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1621–1625. [Google Scholar] [CrossRef] [Green Version]
- Irimie, A.I.; Braicu, C.; Zanoaga, O.; Pileczki, V.; Gherman, C.; Berindan-Neagoe, I.; Campian, R.S. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis and autophagy in oral cancer SSC-4 cells. OncoTargets Ther. 2015, 8, 461–470. [Google Scholar]
- Bauman, J.E.; Zang, Y.; Sen, M.; Li, C.; Wang, L.; Egner, P.A.; Fahey, J.W.; Normolle, D.P.; Grandis, J.R.; Kensler, T.W.; et al. Prevention of Carcinogen-Induced Oral Cancer by Sulforaphane. Cancer Prev. Res. 2016, 9, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Galvão De Podestá, O.P.; Peres, S.V.; Salaroli, L.B.; Cattafesta, M.; De Podestá, J.R.V.; von Zeidler, S.L.V.; de Oliveira, J.C.; Kowalski, L.P.; Ikeda, M.K.; Brennan, P.; et al. Consumption of minimally processed foods as protective factors in the genesis of squamous cell carcinoma of the head and neck in Brazil. PLoS ONE 2019, 14, e0220067. [Google Scholar]
- Green, J.M.; Ciancio, M.J.; Goral, J.; Pytynia, M.; Pitstick, L.; Meyer, A.; Nguyen, A.; Lee, K.; Barakat, A.; Jham, B.C. Dietary fat and male sex increase histopathological changes in a mouse model of oral cancer. Oral Dis. 2021, 27, 215–225. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.D.C.; Puente-Gutiérrez, C.; Delgado-Somolinos, E.; Martín Carreras-Presas, C.; Fernández-Farhall, J.; López-Sánchez, A.F. Association between Oral Cancer and Diet: An Update. Nutrients 2021, 13, 1299. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Negri, E.; Boffetta, P.; La Vecchia, C.; Boyle, P. Mouthwash and oral cancer risk quantitative meta-analysis of epidemiologic studies. Ann. Agric. Environ. Med. 2012, 19, 173–180. [Google Scholar] [PubMed]
- Ustrell-Borràs, M.; Traboulsi-Garet, B.; Gay-Escoda, C. Alcohol-based mouthwash as a risk factor of oral cancer: A systematic review. Med. Oral Patol. Oral Y Cir. Bucal 2020, 25, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Aceves Argemí, R.; González Navarro, B.; Ochoa García-Seisdedos, P.; Estrugo Devesa, A.; López-López, J. Mouthwash With Alcohol and Oral Carcinogenesis: Systematic Review and Meta-analysis. J. Evid. -Based Dent. Pract. 2020, 20, 101407. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Torabi, S.J.; Yarbrough, W.G.; Mehra, S.; Osborn, H.A.; Judson, B. Association of Human Papillomavirus Status at Head and Neck Carcinoma Subsites With Overall Survival. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 519–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [Green Version]
- Devaraja, K.; Aggarwal, S.; Verma, S.S.; Gupta, S.C. Clinico-pathological peculiarities of human papilloma virus driven head and neck squamous cell carcinoma: A comprehensive update. Life Sci. 2020, 245, 117383. [Google Scholar] [CrossRef]
- Lin, N.C.; Hsu, J.T.; Tsai, K.Y. Difference between Female and Male Patients with Oral Squamous Cell Carcinoma: A Single-Center Retrospective Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 3978. [Google Scholar] [CrossRef]
- Auguste, A.; Deloumeaux, J.; Joachim, C.; Gaete, S.; Michineau, L.; Herrmann-Storck, C.; Duflo, S.; Luce, D. Joint effect of tobacco, alcohol, and oral HPV infection on head and neck cancer risk in the French West Indies. Cancer Med. 2020, 9, 6854–6863. [Google Scholar] [CrossRef]
- Nair, S.; Pillai, M.R. Human papillomavirus and disease mechanisms: Relevance to oral and cervical cancers. Oral Dis. 2005, 11, 350–359. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Robayo, D.A.G.; Erira, H.A.T.; Jaimes, F.O.G.; Torres, A.M.; Galindo, A.I.C. Oropharyngeal Squamous Cell Carcinoma: Human Papilloma Virus Coinfection with Streptococcus anginosus. Braz. Dent. J. 2019, 30, 626–633. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Giovane, G.; Kouidhi, S.; Iorio, R.; Romano, M.; De Francesco, F.; Feola, A.; Siciliano, C.; Califano, L.; Giordano, A. HPV epigenetic mechanisms related to Oropharyngeal and Cervix cancers. Cancer Biol. Ther. 2018, 19, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Visalli, G.; Currò, M.; Facciolà, A.; Riso, R.; Mondello, P.; Laganà, P.; Di Pietro, A.; Picerno, I.; Spataro, P. Prevalence of human papillomavirus in saliva of women with HPV genital lesions. Infect. Agents Cancer 2016, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Yen, C.Y.; Lu, M.C.; Tzeng, C.C.; Huang, J.Y.; Chang, H.W.; Chen, R.S.; Liu, S.Y.; Liu, S.T.; Shieh, B.; Li, C. Detection of EBV infection and gene expression in oral cancer from patients in Taiwan by microarray analysis. J. Biomed. Biotechnol. 2009, 2009, 904589. [Google Scholar] [CrossRef]
- Ram, H.; Sarkar, J.; Kumar, H.; Konwar, R.; Bhatt, M.L.; Mohammad, S. Oral cancer: Risk factors and molecular pathogenesis. J. Maxillofac. Oral Surg. 2011, 10, 132–137. [Google Scholar] [CrossRef] [Green Version]
- De Lima, M.A.P.; Teodoro, I.P.P.; Galiza, L.E.; Filho, P.; Marques, F.M.; Pinheiro Junior, R.F.F.; Macedo, G.E.C.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr Virus and Oral Carcinoma: A Systematic Review with Meta-Analysis. Crit. Rev. Oncog. 2019, 24, 349–368. [Google Scholar] [CrossRef]
- Kis, A.; Fehér, E.; Gáll, T.; Tar, I.; Boda, R.; Tóth, E.D.; Méhes, G.; Gergely, L.; Szarka, K. Epstein-Barr virus prevalence in oral squamous cell cancer and in potentially malignant oral disorders in an eastern Hungarian population. Eur. J. Oral Sci. 2009, 117, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Eliopoulos, A.G.; Young, L.S. LMP1 structure and signal transduction. Semin. Cancer Biol. 2001, 11, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G. Cancer at the time of the COVID-19 hurricane. J. Exp. Clin. Cancer Res. 2020, 39, 74. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, N.; Apolone, G.; Botti, G.; Ciliberto, G.; Costantini, M.; De Paoli, P.; Franceschi, S.; Opocher, G.; Paradiso, A.; Pronzato, P.; et al. A moonshot approach toward the management of cancer patients in the COVID-19 time: What have we learned and what could the Italian network of cancer centers (Alliance Against Cancer, ACC) do after the pandemic wave? J. Exp. Clin. Cancer Res. 2020, 39, 109. [Google Scholar] [CrossRef] [PubMed]
- Mariz, B.; Brandão, T.B.; Ribeiro, A.C.P.; Lopes, M.A.; Santos-Silva, A.R. New Insights for the Pathogenesis of COVID-19-Related Dysgeusia. J. Dent. Res. 2020, 99, 1206. [Google Scholar] [CrossRef]
- Brandão, T.B.; Gueiros, L.A.; Melo, T.S.; Prado-Ribeiro, A.C.; Nesrallah, A.; Prado, G.V.B.; Santos-Silva, A.R.; Migliorati, C.A. Oral lesions in patients with SARS-CoV-2 infection: Could the oral cavity be a target organ? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e45–e51. [Google Scholar] [CrossRef]
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Meurman, J.H.; Bascones-Martinez, A. Are oral and dental diseases linked to cancer? Oral Dis. 2011, 17, 779–784. [Google Scholar] [CrossRef]
- Galvão-Moreira, L.V.; da Cruz, M.C. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef]
- Rai, A.K.; Panda, M.; Das, A.K.; Rahman, T.; Das, R.; Das, K.; Sarma, A.; Kataki, A.C.; Chattopadhyay, I. Dysbiosis of salivary microbiome and cytokines influence oral squamous cell carcinoma through inflammation. Arch. Microbiol. 2021, 203, 137–152. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Auluck, A.; Walker, B.B.; Hislop, G.; Lear, S.A.; Schuurman, N.; Rosin, M. Socio-economic deprivation: A significant determinant affecting stage of oral cancer diagnosis and survival. BMC Cancer 2016, 16, 569. [Google Scholar] [CrossRef] [Green Version]
- Hung, L.C.; Kung, P.T.; Lung, C.H.; Tsai, M.H.; Liu, S.A.; Chiu, L.T.; Huang, K.H.; Tsai, W.C. Assessment of the Risk of Oral Cancer Incidence in A High-Risk Population and Establishment of A Predictive Model for Oral Cancer Incidence Using A Population-Based Cohort in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 665. [Google Scholar] [CrossRef] [Green Version]
- Paget-Bailly, S.; Cyr, D.; Luce, D. Occupational exposures to asbestos, polycyclic aromatic hydrocarbons and solvents, and cancers of the oral cavity and pharynx: A quantitative literature review. Int. Arch. Occup. Environ. Health 2012, 85, 341–351. [Google Scholar] [CrossRef]
- Smailyte, G. Cancer incidence among workers exposed to softwood dust in Lithuania. Occup. Environ. Med. 2012, 69, 449–451. [Google Scholar] [CrossRef]
- Hashim, D.; Boffetta, P. Occupational and environmental exposures and cancers in developing countries. Ann. Glob. Health 2014, 80, 393–411. [Google Scholar] [CrossRef]
- Adrien, J.; Bertolus, C.; Gambotti, L.; Mallet, A.; Baujat, B. Why are head and neck squamous cell carcinoma diagnosed so late? Influence of health care disparities and socio-economic factors. Oral Oncol. 2014, 50, 90–97. [Google Scholar] [CrossRef]
- Dholam, K.P.; Chouksey, G.C. Squamous cell carcinoma of the oral cavity and oropharynx in patients aged 18–45 years: A case-control study to evaluate the risk factors with emphasis on stress, diet, oral hygiene, and family history. Indian J. Cancer 2016, 53, 244–251. [Google Scholar]
- Hashim, D.; Sartori, S.; Brennan, P.; Curado, M.P.; Wünsch-Filho, V.; Divaris, K.; Olshan, A.F.; Zevallos, J.P.; Winn, D.M.; Franceschi, S.; et al. The role of oral hygiene in head and neck cancer: Results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann. Oncol. 2016, 27, 1619–1625. [Google Scholar] [CrossRef]
- Kawakita, D.; Lee, Y.A.; Li, Q.; Chen, Y.; Chen, C.J.; Hsu, W.L.; Lou, P.J.; Zhu, C.; Pan, J.; Shen, H.; et al. Impact of oral hygiene on head and neck cancer risk in a Chinese population. Head Neck 2017, 39, 2549–2557. [Google Scholar] [CrossRef]
- Mathur, R.; Singhavi, H.R.; Malik, A.; Nair, S.; Chaturvedi, P. Role of Poor Oral Hygiene in Causation of Oral Cancer-a Review of Literature. Indian J. Surg. Oncol. 2019, 10, 184–195. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Causes of oral cancer—An appraisal of controversies. Br. Dent. J. 2009, 207, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Garavello, W.; Foschi, R.; Talamini, R.; La Vecchia, C.; Rossi, M.; Dal Maso, L.; Tavani, A.; Levi, F.; Barzan, L.; Ramazzotti, V.; et al. Family history and the risk of oral and pharyngeal cancer. Int. J. Cancer 2008, 122, 1827–1831. [Google Scholar] [CrossRef]
- Radoï, L.; Paget-Bailly, S.; Guida, F.; Cyr, D.; Menvielle, G.; Schmaus, A.; Carton, M.; Cénée, S.; Sanchez, M.; Guizard, A.V.; et al. Family history of cancer, personal history of medical conditions and risk of oral cavity cancer in France: The ICARE study. BMC Cancer 2013, 13, 560. [Google Scholar] [CrossRef]
- Brown, L.M.; Gridley, G.; Diehl, S.R.; Winn, D.M.; Harty, L.C.; Otero, E.B.; Fraumeni, J.F., Jr.; Hayes, R.B. Family cancer history and susceptibility to oral carcinoma in Puerto Rico. Cancer 2001, 92, 2102–2108. [Google Scholar] [CrossRef]
- Goldgar, D.E.; Easton, D.F.; Cannon-Albright, L.A.; Skolnick, M.H. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J. Natl. Cancer Inst. 1994, 86, 1600–1608. [Google Scholar] [CrossRef]
- Chen, S.; Lin, Z.; Chen, J.; Yang, A.; Zhang, Q.; Xie, C.; Zhang, X.; Yang, Z.; Chen, W.; Song, M. Older age is a risk factor associated with poor prognosis of patients with squamous cell carcinoma of the oral cavity. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2573–2580. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.J.; Jiang, R.S.; Wu, S.H.; Chen, F.J.; Liu, S.A. Smoking, alcohol, and betel quid and oral cancer: A prospective cohort study. J. Oncol. 2011, 2011, 525976. [Google Scholar] [CrossRef] [PubMed]
- Nosratzehi, T. Salivary Chemical Factors in Relation with Oral Cancer in Smokers and Non-Smokers: A Literature Review. J. Dent. 2017, 18, 237–243. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chi, C.W.; Liu, T.Y. Hydroxyl radical formation and oxidative DNA damage induced by areca quid in vivo. J. Toxicol. Environ. Health Part A 2002, 65, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, C.M.; Shao, Z.; Zhao, X.P.; Wang, M.; Yan, T.L.; Zhou, X.C.; Jiang, E.H.; Liu, K.; Shang, Z.J. Autophagy Induced by Areca Nut Extract Contributes to Decreasing Cisplatin Toxicity in Oral Squamous Cell Carcinoma Cells: Roles of Reactive Oxygen Species/AMPK Signaling. Int. J. Mol. Sci. 2017, 18, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.H.; Kao, S.Y.; Liu, T.Y.; Liu, S.T.; Huang, W.P.; Chang, K.W.; Lin, S.C. Areca nut extract induced oxidative stress and upregulated hypoxia inducing factor leading to autophagy in oral cancer cells. Autophagy 2010, 6, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisky, D.C.; Levy, D.D.; Littlepage, L.E.; Liu, H.; Nelson, C.M.; Fata, J.E.; Leake, D.; Godden, E.L.; Albertson, D.G.; Nieto, M.A.; et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005, 436, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Tsai, C.H.; Yu, C.C.; Chang, Y.C. Elevated snail expression mediates tumor progression in areca quid chewing-associated oral squamous cell carcinoma via reactive oxygen species. PLoS ONE 2013, 8, e67985. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.H.; Hsieh, W.F.; Chiang, W.F.; Hong, W.Z.; Hsu, Y.R.; Cheng, Y.C.; Chen, T.C.; Hsu, K.C.; Lin, P.Y.; Liu, S.Y.; et al. Autophagy induction by the 30–100kDa fraction of areca nut in both normal and malignant cells through reactive oxygen species. Oral Oncol. 2010, 46, 822–828. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Cao, J.Y.; Mansouri, S.; Frappier, L. Changes in the nasopharyngeal carcinoma nuclear proteome induced by the EBNA1 protein of Epstein-Barr virus reveal potential roles for EBNA1 in metastasis and oxidative stress responses. J. Virol. 2012, 86, 382–394. [Google Scholar] [CrossRef] [Green Version]
- Lai, D.; Tan, C.L.; Gunaratne, J.; Quek, L.S.; Nei, W.; Thierry, F.; Bellanger, S. Localization of HPV-18 E2 at mitochondrial membranes induces ROS release and modulates host cell metabolism. PLoS ONE 2013, 8, e75625. [Google Scholar] [CrossRef] [Green Version]
- Williams, V.M.; Filippova, M.; Filippov, V.; Payne, K.J.; Duerksen-Hughes, P. Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J. Virol. 2014, 88, 6751–6761. [Google Scholar] [CrossRef] [Green Version]
- Marullo, R.; Werner, E.; Zhang, H.; Chen, G.Z.; Shin, D.M.; Doetsch, P.W. HPV16 E6 and E7 proteins induce a chronic oxidative stress response via NOX2 that causes genomic instability and increased susceptibility to DNA damage in head and neck cancer cells. Carcinogenesis 2015, 36, 1397–1406. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Gibson, S.B. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell. Signal. 2013, 25, 50–65. [Google Scholar] [CrossRef]
- Gao, L.; Dou, Z.C.; Ren, W.H.; Li, S.M.; Liang, X.; Zhi, K.Q. CircCDR1as upregulates autophagy under hypoxia to promote tumor cell survival via AKT/ERK(1/2)/mTOR signaling pathways in oral squamous cell carcinomas. Cell Death Dis. 2019, 10, 745. [Google Scholar] [CrossRef] [Green Version]
- Philibert, R.; Erwin, C. A Review of Epigenetic Markers of Tobacco and Alcohol Consumption. Behav. Sci. Law 2015, 33, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Hutchison, K.E.; Bryan, A.D.; Filbey, F.M.; Calhoun, V.D.; Claus, E.D.; Lin, D.; Sui, J.; Du, Y.; Liu, J. Opposite Epigenetic Associations With Alcohol Use and Exercise Intervention. Front. Psychiatry 2018, 9, 594. [Google Scholar] [CrossRef]
- Sabi, S.H.; Khabour, O.F.; Alzoubi, K.H.; Cobb, C.O.; Eissenberg, T. Changes at global and site-specific DNA methylation of MLH1 gene promoter induced by waterpipe smoking in blood lymphocytes and oral epithelial cells. Inhal. Toxicol. 2020, 32, 124–130. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, S.; Maitra, A.; Biswas, N.K.; Panda, C.K.; Roy, B.; Sarin, R.; Majumder, P.P. Epigenomic dysregulation-mediated alterations of key biological pathways and tumor immune evasion are hallmarks of gingivo-buccal oral cancer. Clin. Epigenetics 2019, 11, 178. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Behm-Ansmant, I.; Rehwinkel, J.; Izaurralde, E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Irimie, A.I.; Zimta, A.A.; Ciocan, C.; Mehterov, N.; Dudea, D.; Braicu, C.; Berindan-Neagoe, I. The Unforeseen Non-Coding RNAs in Head and Neck Cancer. Genes 2018, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Tomuleasa, C.; Braicu, C.; Irimie, A.; Craciun, L.; Berindan-Neagoe, I. Nanopharmacology in translational hematology and oncology. Int. J. Nanomed. 2014, 9, 3465–3479. [Google Scholar]
- Nagadia, R.; Pandit, P.; Coman, W.B.; Cooper-White, J.; Punyadeera, C. miRNAs in head and neck cancer revisited. Cell. Oncol. 2013, 36, 1–7. [Google Scholar] [CrossRef]
- Chu, A.; Robertson, G.; Brooks, D.; Mungall, A.J.; Birol, I.; Coope, R.; Ma, Y.; Jones, S.; Marra, M.A. Large-scale profiling of microRNAs for The Cancer Genome Atlas. Nucleic Acids Res. 2016, 44, e3. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Sonea, L.; Zimta, A.A.; Cojocneanu-Petric, R.; Tonchev, K.; Mehterov, N.; Diudea, D.; Buduru, S.; Berindan-Neagoe, I. A Looking-Glass of Non-coding RNAs in oral cancer. Int. J. Mol. Sci. 2017, 18, 2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander-Dann, B.; Pruteanu, L.L.; Oerton, E.; Sharma, N.; Berindan-Neagoe, I.; Módos, D.; Bender, A. Developments in toxicogenomics: Understanding and predicting compound-induced toxicity from gene expression data. Mol. Omics 2018, 14, 218–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiano, G.; Boldrup, L.; Ardito, F.; Gu, X.; Lo Muzio, L.; Nylander, K. Circulating miRNAs from blood, plasma or serum as promising clinical biomarkers in oral squamous cell carcinoma: A systematic review of current findings. Oral Oncol. 2016, 63, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lv, X.; Lv, X.; Ma, Y.; Chen, L.; Chen, Y. Circulating miR-21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget 2017, 8, 44050–44058. [Google Scholar] [CrossRef] [Green Version]
- Osan, C.; Chira, S.; Nutu, A.M.; Braicu, C.; Baciut, M.; Korban, S.S.; Berindan-Neagoe, I. The Connection between MicroRNAs and Oral Cancer Pathogenesis: Emerging Biomarkers in Oral Cancer Management. Genes 2021, 12, 1989. [Google Scholar] [CrossRef]
- Irimie, A.I.; Sonea, L.; Jurj, A.; Mehterov, N.; Zimta, A.A.; Budisan, L.; Braicu, C.; Berindan-Neagoe, I. Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int. J. Nanomed. 2017, 12, 4593–4606. [Google Scholar] [CrossRef] [Green Version]
- Bersani, C.; Mints, M.; Tertipis, N.; Haeggblom, L.; Näsman, A.; Romanitan, M.; Dalianis, T.; Ramqvist, T. MicroRNA-155, -185 and -193b as biomarkers in human papillomavirus positive and negative tonsillar and base of tongue squamous cell carcinoma. Oral Oncol. 2018, 82, 8–16. [Google Scholar] [CrossRef]
- Fang, C.; Li, Y. Prospective applications of microRNAs in oral cancer. Oncol. Lett. 2019, 18, 3974–3984. [Google Scholar]
- Wu, M.; Duan, Q.; Liu, X.; Zhang, P.; Fu, Y.; Zhang, Z.; Liu, L.; Cheng, J.; Jiang, H. MiR-155-5p promotes oral cancer progression by targeting chromatin remodeling gene ARID2. Biomed. Pharmacother. 2020, 122, 109696. [Google Scholar] [CrossRef]
- Manikandan, M.; Deva Magendhra Rao, A.K.; Rajkumar, K.S.; Rajaraman, R.; Munirajan, A.K. Altered levels of miR-21, miR-125b-2*, miR-138, miR-155, miR-184, and miR-205 in oral squamous cell carcinoma and association with clinicopathological characteristics. J. Oral Pathol. Med. 2015, 44, 792–800. [Google Scholar] [CrossRef]
- Lajer, C.B.; Nielsen, F.C.; Friis-Hansen, L.; Norrild, B.; Borup, R.; Garnæs, E.; Rossing, M.; Specht, L.; Therkildsen, M.H.; Nauntofte, B.; et al. Different miRNA signatures of oral and pharyngeal squamous cell carcinomas: A prospective translational study. Br. J. Cancer 2011, 104, 830–840. [Google Scholar] [CrossRef]
- Lopes, C.B.; Magalhães, L.L.; Teófilo, C.R.; Alves, A.; Montenegro, R.C.; Negrini, M.; Ribeiro-Dos-Santos, Â. Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer. BMC Cancer 2018, 18, 721. [Google Scholar] [CrossRef]
- Jia, B.; Zhang, S.; Wu, S.; Zhu, Q.; Li, W. MiR-770 promotes oral squamous cell carcinoma migration and invasion by regulating the Sirt7/Smad4 pathway. IUBMB Life 2021, 73, 264–272. [Google Scholar] [CrossRef]
- Rajan, C.; Roshan, V.G.D.; Khan, I.; Manasa, V.G.; Himal, I.; Kattoor, J.; Thomas, S.; Kondaiah, P.; Kannan, S. MiRNA expression profiling and emergence of new prognostic signature for oral squamous cell carcinoma. Sci. Rep. 2021, 11, 7298. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yu, S.K.; Lee, M.H.; Park, M.G.; Park, E.; Kim, S.G.; Lee, S.Y.; Kim, C.S.; Kim, H.J.; Chun, H.S.; et al. MicroRNA-205 directly regulates the tumor suppressor, interleukin-24, in human KB oral cancer cells. Mol. Cells 2013, 35, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozaki, K.; Imoto, I.; Mogi, S.; Omura, K.; Inazawa, J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008, 68, 2094–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uesugi, A.; Kozaki, K.; Tsuruta, T.; Furuta, M.; Morita, K.; Imoto, I.; Omura, K.; Inazawa, J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011, 71, 5765–5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiah, S.G.; Hsiao, J.R.; Chang, W.M.; Chen, Y.W.; Jin, Y.T.; Wong, T.Y.; Huang, J.S.; Tsai, S.T.; Hsu, Y.M.; Chou, S.T.; et al. Downregulated miR329 and miR410 promote the proliferation and invasion of oral squamous cell carcinoma by targeting Wnt-7b. Cancer Res. 2014, 74, 7560–7572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, G.; Fazzari, M.; Kung, G.; Kawachi, N.; Brandwein-Gensler, M.; McLemore, M.; Chen, Q.; Burk, R.D.; Smith, R.V.; Prystowsky, M.B.; et al. Low-level expression of microRNAs let-7d and miR-205 are prognostic markers of head and neck squamous cell carcinoma. Am. J. Pathol. 2009, 174, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Kao, Y.Y.; Chou, C.H.; Yeh, L.Y.; Chen, Y.F.; Chang, K.W.; Liu, C.J.; Fan Chiang, C.Y.; Lin, S.C. MicroRNA miR-31 targets SIRT3 to disrupt mitochondrial activity and increase oxidative stress in oral carcinoma. Cancer Lett. 2019, 456, 40–48. [Google Scholar] [CrossRef]
- Endo, H.; Muramatsu, T.; Furuta, M.; Uzawa, N.; Pimkhaokham, A.; Amagasa, T.; Inazawa, J.; Kozaki, K. Potential of tumor-suppressive miR-596 targeting LGALS3BP as a therapeutic agent in oral cancer. Carcinogenesis 2013, 34, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Bhat, M.Y.; Advani, J.; Rajagopalan, P.; Patel, K.; Nanjappa, V.; Solanki, H.S.; Patil, A.H.; Bhat, F.A.; Mathur, P.P.; Nair, B.; et al. Cigarette smoke and chewing tobacco alter expression of different sets of miRNAs in oral keratinocytes. Sci. Rep. 2018, 8, 7040. [Google Scholar] [CrossRef]
- Shiah, S.G.; Hsiao, J.R.; Chang, H.J.; Hsu, Y.M.; Wu, G.H.; Peng, H.Y.; Chou, S.T.; Kuo, C.C.; Chang, J.Y. MiR-30a and miR-379 modulate retinoic acid pathway by targeting DNA methyltransferase 3B in oral cancer. J. Biomed. Sci. 2020, 27, 46. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.Y.; Hsiao, J.R.; Chou, S.T.; Hsu, Y.M.; Wu, G.H.; Shieh, Y.S.; Shiah, S.G. MiR-944/CISH mediated inflammation via STAT3 is involved in oral cancer malignance by cigarette smoking. Neoplasia 2020, 22, 554–565. [Google Scholar] [CrossRef]
- Arunkumar, G.; Deva Magendhra Rao, A.K.; Manikandan, M.; Prasanna Srinivasa Rao, H.; Subbiah, S.; Ilangovan, R.; Murugan, A.K.; Munirajan, A.K. Dysregulation of miR-200 family microRNAs and epithelial-mesenchymal transition markers in oral squamous cell carcinoma. Oncol. Lett. 2018, 15, 649–657. [Google Scholar] [CrossRef]
- Krishnan, A.R.; Zheng, H.; Kwok, J.G.; Qu, Y.; Zou, A.E.; Korrapati, A.; Li, P.X.; Califano, J.A.; Hovell, M.F.; Wang-Rodriguez, J.; et al. A comprehensive study of smoking-specific microRNA alterations in head and neck squamous cell carcinoma. Oral Oncol. 2017, 72, 56–64. [Google Scholar] [CrossRef]
- Avissar, M.; McClean, M.D.; Kelsey, K.T.; Marsit, C.J. MicroRNA expression in head and neck cancer associates with alcohol consumption and survival. Carcinogenesis 2009, 30, 2059–2063. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, M.; Deva Magendhra Rao, A.K.; Arunkumar, G.; Rajkumar, K.S.; Rajaraman, R.; Munirajan, A.K. Down Regulation of miR-34a and miR-143 May Indirectly Inhibit p53 in Oral Squamous Cell Carcinoma: A Pilot Study. Asian Pac. J. Cancer Prev. 2015, 16, 7619–7625. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.T.; Peng, H.Y.; Mo, K.C.; Hsu, Y.M.; Wu, G.H.; Hsiao, J.R.; Lin, S.F.; Wang, H.D.; Shiah, S.G. MicroRNA-486-3p functions as a tumor suppressor in oral cancer by targeting DDR1. J. Exp. Clin. Cancer Res. 2019, 38, 281. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Lin, C.S.; Chiang, S.L.; Lee, C.H.; Lee, K.W.; Ko, Y.C. Areca nut induces miR-23a and inhibits repair of DNA double-strand breaks by targeting FANCG. Toxicol. Sci. 2011, 123, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Chuerduangphui, J.; Ekalaksananan, T.; Chaiyarit, P.; Patarapadungkit, N.; Chotiyano, A.; Kongyingyoes, B.; Promthet, S.; Pientong, C. Effects of arecoline on proliferation of oral squamous cell carcinoma cells by dysregulating c-Myc and miR-22, directly targeting oncostatin M. PLoS ONE 2018, 13, e0192009. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Srivastava, A.N.; Sharma, R.; Mateen, S.; Shukla, B.; Singh, A.; Chandel, S. Circulating MicroRNA-21 Expression as a Novel Serum Biomarker for Oral Sub-Mucous Fibrosis and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 1053–1057. [Google Scholar]
- Cao, M.X.; Zhang, W.L.; Yu, X.H.; Wu, J.S.; Qiao, X.W.; Huang, M.C.; Wang, K.; Wu, J.B.; Tang, Y.J.; Jiang, J.; et al. Interplay between cancer cells and M2 macrophages is necessary for miR-550a-3-5p down-regulation-mediated HPV-positive OSCC progression. J. Exp. Clin. Cancer Res. 2020, 39, 102. [Google Scholar] [CrossRef]
- Božinović, K.; Sabol, I.; Dediol, E.; Milutin Gašperov, N.; Manojlović, S.; Vojtechova, Z.; Tachezy, R.; Grce, M. Genome-wide miRNA profiling reinforces the importance of miR-9 in human papillomavirus associated oral and oropharyngeal head and neck cancer. Sci. Rep. 2019, 9, 2306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Hu, S.Q.; Pu, Y.M.; Zhang, K.; Wang, Y.X. A HPV16-related prognostic indicator for head and neck squamous cell carcinoma. Ann. Transl. Med. 2020, 8, 1492. [Google Scholar] [CrossRef]
- House, R.; Majumder, M.; Janakiraman, H.; Ogretmen, B.; Kato, M.; Erkul, E.; Hill, E.; Atkinson, C.; Barth, J.; Day, T.A.; et al. Smoking-induced control of miR-133a-3p alters the expression of EGFR and HuR in HPV-infected oropharyngeal cancer. PLoS ONE 2018, 13, e0205077. [Google Scholar] [CrossRef] [Green Version]
- Lepore, S.; Lettini, G.; Condelli, V.; Sisinni, L.; Piscazzi, A.; Simeon, V.; Zoppoli, P.; Pedicillo, M.C.; Natalicchio, M.I.; Pietrafesa, M.; et al. Comparative Gene Expression Profiling of Tobacco-Associated HPV-Positive versus Negative Oral Squamous Carcinoma Cell Lines. Int. J. Med. Sci. 2020, 17, 112–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacconi, A.; Donzelli, S.; Pulito, C.; Ferrero, S.; Spinella, F.; Morrone, A.; Rigoni, M.; Pimpinelli, F.; Ensoli, F.; Sanguineti, G.; et al. TMPRSS2, a SARS-CoV-2 internalization protease is downregulated in head and neck cancer patients. J. Exp. Clin. Cancer Res. 2020, 39, 200. [Google Scholar] [CrossRef]
- Cai, L.; Ye, Y.; Jiang, Q.; Chen, Y.; Lyu, X.; Li, J.; Wang, S.; Liu, T.; Cai, H.; Yao, K.; et al. Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat. Commun. 2015, 6, 7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Long, Y.; Chong, T.; Cai, W.; Tsang, C.M.; Zhou, X.; Lin, Y.; Ding, T.; Zhou, W.; Zhao, H.; et al. EBV-miR-BART7-3p Imposes Stemness in Nasopharyngeal Carcinoma Cells by Suppressing SMAD7. Front. Genet. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taş, B.; Güre, A.O. The effect of Maras powder and smoking on the microRNA deregulation of oral mucosa. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190382. [Google Scholar] [CrossRef] [Green Version]
- Rishabh, K.; Khadilkar, S.; Kumar, A.; Kalra, I.; Kumar, A.P.; Kunnumakkara, A.B. MicroRNAs as Modulators of Oral Tumorigenesis-A Focused Review. Int. J. Mol. Sci. 2021, 22, 2561. [Google Scholar] [CrossRef]
- Paluszczak, J. The Significance of the Dysregulation of Canonical Wnt Signaling in Head and Neck Squamous Cell Carcinomas. Cells 2020, 9, 723. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhou, Y.; Zhang, L.; Chen, Y.; Lyu, X.; Cai, L.; Lu, Y.; Deng, Y.; Wang, J.; Yao, K.; et al. EBV-miR-BART1 is involved in regulating metabolism-associated genes in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2013, 436, 19–24. [Google Scholar] [CrossRef] [PubMed]


| Risk Factor | Tumor Type | miR | Targets | Effects/Clinical Significance | References |
|---|---|---|---|---|---|
| smoking | OSCC | miR-31-5p↑ | SLC16A1 | Cancer cell proliferation | [199] |
| OSCC | miR-30a↓ miR-379↓ | DNMT3B | Growth inhibition in oral cancer cells | [200] | |
| OSCC | miR-944↑ | CISH STAT3 | Maintaining a pro-carcinogenic microenvironment in oral cancer | [201] | |
| OSCC | miR-200a, miR-200b, miR-200c, miR-141 and miR-429, ↓ | ZEB2-AS1 and ZEB2 | No significant effect on treatment outcome | [202] | |
| HNSCC | miR-101-1, miR-181b-1, miR-486, and miR-1301↑ | Increase of cell proliferation, metastasis, and decrease in survival | [203] | ||
| alcohol | HNSCC | miR-375↑ | Decrease in survival | [204] | |
| OSCC | miR-34a↓ | P53 | Inhibition of tumor growth | [205] | |
| miR-30a↑miR-934↑ | BCL-2 | Increase in cellular proliferation | [88] | ||
| betel/tobacco chewing | OSCC | miR-155↑ | Increase in cellular proliferation | [187] | |
| OSCC | miR-486-3p↓ | DDR1 | Growth inhibition and apoptosis induction | [206] | |
| OSCC | miR-30a↓ miR-379↓ | DNMT3B | Growth inhibition in oral cancer cells | [200] | |
| OSCC | miR-29c-3p miR-146a-5p↑ | SLC2A14 STAT 1, MX2, OASL | Cancer cells proliferation | [199] | |
| OSCC | miR329 and miR410↓ | Wnt-7b | Proliferation and invasion of cells | [195] | |
| Areca nut | OSCC | miR-23a↑ | FANCG | Induction of cell proliferation | [207] |
| OSCC | miR-22↓ | OSM | Promote cell proliferation and cell-cycle progression | [208] | |
| pan-masala chewing | OSCC | miR-21↑ | Poor prognosis | [209] | |
| HPV | TSCC/BOTSCC | miR-155↑ miR-193b↓ miR-185↑ | CD8+ TIL | Decreased survival | [184] |
| OSCC | miR-550a-3-5p↓ | YAP CCL2 | Larger tumor size and nodal metastasis | [210] | |
| HNSCC | miR-9↑ | Proliferation and migration of the cells | [211] | ||
| HNSCC | miR-99a-3p and miR-4746-5p↑ miR-411-5p↓ | MAPK FoxO | Improvement of overall survival | [212] | |
| OPSCC | miR-133a-3p↓ | EGFR and HuR | Promote cell proliferation | [213] | |
| OSCC | let-7e↑ | βCatenin | Induction of stem-like traits in tobacco-related OSCCs | [214] | |
| SARS-CoV-2 | HNSCC | miR-193b-3p; miR-503-5p; miR-455-5p; miR-31-3p; miR-193b-5p; miR-2355-5p↑ | TMPRSS2 | Resistance to SARS-CoV-2 infection | [215] |
| EBV | NPC | EBV-miR-BART1↑ | G6PD, SAT1, ASS1, PAST1, FUT1, SGPL1, DHRS3, PHGDH, GALNT1 | Tumor metastasis | [216] |
| NPC | miR-BART7-3p↑ | SMAD7 | Drug resistance and cancer recurrence | [217] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghiorghiesei, O.; Zanoaga, O.; Nutu, A.; Braicu, C.; Campian, R.S.; Lucaciu, O.; Berindan Neagoe, I. The World of Oral Cancer and Its Risk Factors Viewed from the Aspect of MicroRNA Expression Patterns. Genes 2022, 13, 594. https://doi.org/10.3390/genes13040594
Aghiorghiesei O, Zanoaga O, Nutu A, Braicu C, Campian RS, Lucaciu O, Berindan Neagoe I. The World of Oral Cancer and Its Risk Factors Viewed from the Aspect of MicroRNA Expression Patterns. Genes. 2022; 13(4):594. https://doi.org/10.3390/genes13040594
Chicago/Turabian StyleAghiorghiesei, Ovidiu, Oana Zanoaga, Andreea Nutu, Cornelia Braicu, Radu Septimiu Campian, Ondine Lucaciu, and Ioana Berindan Neagoe. 2022. "The World of Oral Cancer and Its Risk Factors Viewed from the Aspect of MicroRNA Expression Patterns" Genes 13, no. 4: 594. https://doi.org/10.3390/genes13040594
APA StyleAghiorghiesei, O., Zanoaga, O., Nutu, A., Braicu, C., Campian, R. S., Lucaciu, O., & Berindan Neagoe, I. (2022). The World of Oral Cancer and Its Risk Factors Viewed from the Aspect of MicroRNA Expression Patterns. Genes, 13(4), 594. https://doi.org/10.3390/genes13040594