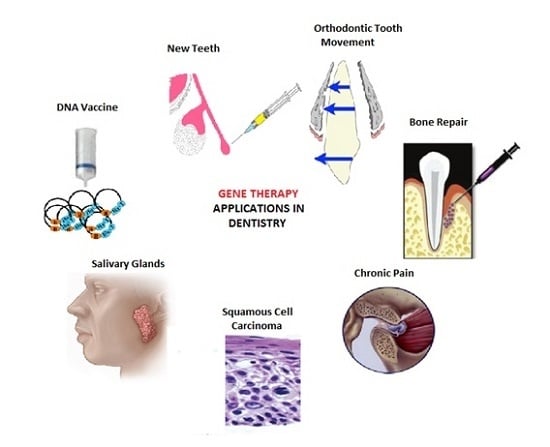
Gene Therapy: A Paradigm Shift in Dentistry
Abstract
:1. Introduction
2. Historical Background
3. Applications in Dentistry
3.1. Orofacial Pain
3.2. Carcinomas
- Corrective gene therapy (Gene Replacement Therapy) involves correction of the underlying genetic defect to control the unrestricted multiplication of tumor cells. The over-expressed oncogenes are blocked or silenced by inclusion of DNA into the cell, while successfully disrupting the processes of transcription and translation. It is also worth mentioning that the mutation or inactivation of the p53 gene is seen in the infancy of many tumors, due to which the defensive mechanism of apoptosis is disabled and the abnormal cells are free to multiply. With gene therapy, the correct copy of p53 gene is introduced into the tumor cells leading to apoptosis. At the same time, this makes the tumor cells more receptive to radiotherapy [41].
- In 2003, the first gene therapy drug Gendicine, a recombinant human adenovirus p53, was formulated and approved by the Chinese authorities for the treatment of SCCHN and other cancerous lesions. In a randomized clinical trial, 135 SCHNN patients were treated with radiotherapy alone or in combination with Gendicine. The majority of patients (64%) receiving both Gendicine and radiotherapy exhibited complete remission unlike the radiotherapy only group that showed remission ~19% [26,47,48].
- Cytoreductive gene therapy aims at destruction of tumor cells in multiple ways—for example, through the insertion of suicide genes into tumor cells which encode for enzymes that convert the chemotherapeutic drugs into their toxic form [7]. The introduction of genes can also limit angiogenesis and increase apoptosis in tumor cells .The highlight of this approach, however, are the oncolytic viruses which selectively multiply in tumor cells and kill them. These can significantly reduce the size of the tumor after its surgical removal and prevent metastasis [7]. In 2005, the first genetically engineered oncolytic virus, H101 Adenovirus, was approved for treatment of SCCHN in China [49]. Advanced stage cancer patients showed a 79% response rate with both chemotherapy and the modified adenovirus, as compared to a 40% response rate with chemotherapy alone [50]. These results suggested the promising outcome of using gene therapy as an adjunct for the management of cancers.
- Another approach aims at the modification of the immune system. This is designed to boost up a host′s immune system by injecting genetically modified hematopoietic stem cells and T cells that are highly efficient in identifying and killing tumor cells. This may be coupled by insertion of a gene in tumor cells to upregulate their antigen markers and make them more susceptible to destruction by the body′s own immune system. The concentration of cytokines in tumor cells may also be increased by insertion of a gene encoding for cytokines. Immunotherapy may be beneficial in treatment of SCCHN, melanoma, lymphoma and some virus induced malignancies [51].
3.3. Bone Repair
3.4. Salivary Glands
3.5. Orthodontic Tooth Movements
3.6. Tooth Repair and Regeneration
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Misra, S. Human gene therapy: A brief overview of the genetic revolution. J. Assoc. Physicians India 2013, 61, 127–133. [Google Scholar] [PubMed]
- Wirth, T.; Parker, N.; Ylä-Herttuala, S. History of gene therapy. Gene 2013, 525, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Paul, J.M.; Basappa, N. Gene Therapy and its Implications in Dentistry. Int. J. Clin. Pediatr. Dent. 2011, 4, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Pradeep, A. Gene therapy in periodontics: A review and future implications. J. Contemp. Dent. Pract. 2006, 7, 83–91. [Google Scholar] [PubMed]
- Coutelle, C.; Themis, M.; Waddington, S.; Buckley, S.; Gregory, L.; Nivsarkar, M.; David, A.; Peebles, D.; Weisz, B.; Rodeck, C. Gene therapy progress and prospects: Fetal gene therapy—First proofs of concept—Some adverse effects. Gene Ther. 2005, 12, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Pacilio, C.; Giordano, A. Gene transfer technology in therapy: Current applications and future goals. Stem Cells 1999, 17, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Barbellido, S.A.; Trapero, J.C.; Sánchez, J.C.; García, M.A.P.; Castaño, N.E.; Martínez, A.B. Gene therapy in the management of oral cancer: Review of the literature. Med. Oral Patol. Oral Cir. Bucal 2008, 13, E15–E21. [Google Scholar]
- Vitolo, J.; Baum, B. The use of gene transfer for the protection and repair of salivary glands. Oral Dis. 2002, 8, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.N.; Wu, H.; Shankar, P. Recent advances in RNAi-based strategies for therapy and prevention of HIV-1/AIDS. Adv. Drug Deliv. Rev. 2016, 103, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Tani, K. Current status and recent advances of gene therapy in hematological diseases. Int. J. Hematol. 2016, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Terao, C.; Raychaudhuri, S.; Gregersen, P.K. Recent Advances in Defining the Genetic Basis of Rheumatoid Arthritis. Ann. Rev. Genomics Hum. Genet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ingham, S.A.; Harkins, K.A.; Do, D.V.; Nguyen, Q.D. The role of pharmacogenetics and advances in gene therapy in the treatment of diabetic retinopathy. Pharmacogenomics 2016, 17, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.R.; Suzuki, M. Recent advances in oncolytic adenovirus therapies for cancer. Curr. Opin. Virol. 2016, 21, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, A.C.; Ludwig, M.L.; Spector, M.E.; Brenner, J.C. The potential for tumor suppressor gene therapy in head and neck cancer. Discovery Med. 2016, 21, 41–47. [Google Scholar]
- Chadha, A.S.; Khoo, A.; Aliru, M.L.; Arora, H.K.; Gunther, J.R.; Krishnan, S. Recent Advances and Prospects for Multi-Modality Therapy in Pancreatic Cancer. Semin. Radiat. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Porada, G.; Atala, A.; Porada, C.D. In utero stem cell transplantation and gene therapy: Rationale, history, and recent advances toward clinical application. Mol. Ther. Methods Clin. Dev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Griffith, F. The significance of pneumococcal types. J. Hyg. 1928, 27, 113–159. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; Macleod, C.M.; McCarty, M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types: Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type Iii. J. Exp. Med. 1944, 79, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Zinder, N.D.; Lederberg, J. Genetic exchange in Salmonella. J. Bacteriol. 1952, 64, 679–699. [Google Scholar] [PubMed]
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Temin, H.M. Mixed infection with two types of Rous sarcoma virus. Virology 1961, 13, 158–163. [Google Scholar] [CrossRef]
- Szybalska, E.H.; Szybalski, W. Genetics of human cell line, IV. DNA-mediated heritable transformation of a biochemical trait. Proc. Natl. Acad. Sci. USA. 1962, 48, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Lowenthal, A.; Terheggen, H.G.; Columbo, J.P. Induction of arginase activity with the Shope papilloma virus in tissue culture cells from an argininemic patient. J. Exp. Med. 1973, 137, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F.; Tenen, D.G.; Ackerman, S.J. Molecular cloning of the human eosinophil-derived neurotoxin: A member of the ribonuclease gene family. Proc. Natl. Acad. Sci. USA. 1989, 86, 4460–4464. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, S.G. The biotech death of Jesse Gelsinger. N. Y. Times Mag. 1999, 28, 136–140. [Google Scholar]
- Peng, Z. Current status of gendicine in China: Recombinant human Ad-p53 agent for treatment of cancers. Hum. Gene Ther. 2005, 16, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Gendicine: The first commercial gene therapy product; Chinese translation of editorial. Hum. Gene Ther. 2005, 16, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Hedman, M.; Makinen, K.; Manninen, H.; Immonen, A.; Vapalahti, M.; Yla-Herttuala, S. Safety profile of plasmid/liposomes and virus vectors in clinical gene therapy. Curr. Drug Saf. 2006, 1, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; O’Connell, B.C. The impact of gene therapy on dentistry. J. Am. Dent. Assoc. 1995, 126, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Camu, F.; Vanlersberghe, C. Pharmacology of systemic analgesics. Best Pract. Res. Clin. Anaesthesiol. 2002, 16, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Wang, Y.; Jian, F.; Liao, L.; Yang, X.; Lai, W. Current advances in orthodontic pain. Int. J.Oral Sci. 2016, 8, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Gazal, G.; Fareed, W.M.; Zafar, M.S.; Al-Samadani, K.H. Pain and anxiety management for pediatric dental procedures using various combinations of sedative drugs: A review. Saudi Pharm. J. 2016, 24, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jain, K. Gene therapy for pain. Expert Opin. Biolo. Ther. 2008, 8, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Fink, D.J. A new player in gene therapy for pain? Gene Ther. 2008, 15, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.P.; Yeomans, D.C. Genetic therapy for pain management. Curr. Rev. Pain 2000, 4, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, C.; Yoder, W.E.; Westlund, K.N.; Carlson, C.R.; Miller, C.S.; Danaher, R.J. Efficacy of Herpes Simplex Virus Vector Encoding the Human Preproenkephalin Gene for Treatment of Facial Pain in Mice. J. Oral Facial Pain Headache 2016, 30, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Tzabazis, A.Z.; Klukinov, M.; Feliciano, D.P.; Wilson, S.P.; Yeomans, D.C. Gene therapy for trigeminal pain in mice. Gene Ther. 2014, 21, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, T.; Nakanishi, T.; Kanyama, M.; Sonoyama, W.; Fujisawa, T.; Kobayashi, K.; Ikeda, T.; Kubo, T.; Yamashita, A.; Takigawa, M. Direct adenovirus-mediated gene delivery to the temporomandibular joint in guinea-pigs. Arch. Oral Biol. 1999, 44, 701–709. [Google Scholar] [CrossRef]
- Scully, C.; Felix, D. Oral Medicine—Update for the dental practitioner Orofacial pain. Br. Dent. J. 2006, 200, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, E.; Bapat, U.; Chisholm, C.; Alusi, G.; Vassaux, G. Gene therapy in head and neck cancer: A review. Postgrad. Med. J. 2007, 83, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Chandra, S.; Raj, V.; Fareed, W.; Zafar, M. Molecular and genetic aspects of odontogenic tumors: A review. Iran. J.Basic Med. Sci. 2015, 18, 529–536. [Google Scholar] [PubMed]
- Gleich, L.L.; Salamone, F.N. Molecular genetics of head and neck cancer. Cancer Control 2002, 9, 369–378. [Google Scholar] [PubMed]
- Groot-Wassink, T.; Barthel, H.; Lemoine, N.R.; Vassaux, G. Sodium iodide symporter: A new strategy to target cancer? Lancet 2003, 361, 1905–1906. [Google Scholar] [CrossRef]
- Edelstein, M.L.; Abedi, M.R.; Wixon, J. Gene therapy clinical trials worldwide to 2007—An update. J. Gene Med. 2007, 9, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Vattemi, E.; Claudio, P.P. Adenoviral gene therapy in head and neck cancer. Drug News Perspect. 2006, 19, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Xiao, S.W.; Liu, C.Q.; Sun, Y.; Su, X.; Li, D.M.; Xu, G.; Cai, Y.; Zhu, G.Y.; Xu, B.; et al. Treatment of head and neck squamous cell carcinoma by recombinant adenovirus-p53 combined with radiotherapy: A phase II clinical trial of 42 cases. Zhonghua Yi Xue Za Zhi 2003, 83, 2023–2028. [Google Scholar] [PubMed]
- Chen, C.B.; Pan, J.J.; Xu, L.Y. Recombinant adenovirus p53 agent injection combined with radiotherapy in treatment of nasopharyngeal carcinoma: A phase II clinical trial. Zhonghua Yi Xue Za Zhi 2003, 83, 2033–2035. [Google Scholar] [PubMed]
- Liu, T.; Kirn, D. Gene therapy progress and prospects cancer: Oncolytic viruses. Gene Ther. 2008, 15, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Goldufsky, J.; Sivendran, S.; Harcharik, S.; Pan, M.; Bernardo, S.; Stern, R. Oncolytic virus therapy for cancer. Oncolytic Virother. 2013, 2, 31–46. [Google Scholar] [PubMed]
- Kershaw, M.H.; Teng, M.W.; Smyth, M.J.; Darcy, P.K. Supernatural T cells: Genetic modification of T cells for cancer therapy. Nature Rev. Immunol. 2005, 5, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Kneser, U.; Schaefer, D.J.; Polykandriotis, E.; Horch, R.E. Tissue engineering of bone: The reconstructive surgeon′s point of view. J. Cell Mol. Med. 2006, 10, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Luo, J.; Sun, M.H.; Kang, Q.; Peng, Y.; Jiang, W.; Luu, H.H.; Luo, Q.; Park, J.Y.; Li, Y.; Haydon, R.C. Gene therapy for bone regeneration. Curr. Gene Ther. 2005, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, M.; Imamura, T.; Miyazono, K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998, 9, 49–61. [Google Scholar] [CrossRef]
- Kirker-Head, C.A. Potential applications and delivery strategies for bone morphogenetic proteins. Adv. Drug Deliv. Rev. 2000, 43, 65–92. [Google Scholar] [CrossRef]
- Alden, T.D.; Beres, E.J.; Laurent, J.S.; Engh, J.A.; Das, S.; London, S.D.; Jane, J.A., Jr.; Hudson, S.B.; Helm, G.A. The use of bone morphogenetic protein gene therapy in craniofacial bone repair. J. Craniofac. Surg. 2000, 11, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Kok, M.; Tran, S.D.; Yamano, S. The impact of gene therapy on dentistry: A revisiting after six years. J. Am. Dent. Assoc. 2002, 133, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Gerstenfeld, L.C.; Einhorn, T.A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Anusaksathien, O.; Webb, S.A.; Printz, M.A.; Giannobile, W.V. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol. Ther. 2004, 9, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Bouleftour, W.; Juignet, L.; Bouet, G.; Granito, R.N.; Vanden-Bossche, A.; Laroche, N.; Aubin, J.E.; Lafage-Proust, M.; Vico, L.; Malaval, L. The role of the SIBLING, Bone Sialoprotein in skeletal biology—Contribution of mouse experimental genetics. Matrix Biol. 2016, 52, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Ten Cate's Oral Histology: Development, Structure, and Function; Mosby: St. Louis, MO, USA, 2012. [Google Scholar]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and its applications in dentistry. Saudi Pharm. J. 2016. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral Antimicrobial Peptides: Types and Role in the Oral Cavity. Saudi Pharm. J. 2015, 24, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Rehman, R.; Rehman, I.U. Advances of Proteomic Sciences in Dentistry. Int. J.Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kagami, H.; Wang, S.; Hai, B. Restoring the function of salivary glands. Oral Dis. 2008, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA. 1997, 94, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Yin, H.; Cabrera-Pérez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.D.; Zheng, C.; et al. Aquaporin gene therapy corrects Sjögren′s syndrome phenotype in mice. Pro. Natl. Acad. Sc. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Mastrangeli, A.; O’Connell, B.; Aladib, W.; Fox, P.C.; Baum, B.J.; Crystal, R.G. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am. J. Physiol. 1994, 266, G1146–G1155. [Google Scholar] [PubMed]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA. 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zourelias, L.; Wu, C.; Edwards, P.C.; Trombetta, M.; Passineau, M. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015, 22, 739–749. [Google Scholar] [CrossRef] [PubMed]
- O′Connell, B.C.; Xu, T.; Walsh, T.J.; Sein, T.; Mastrangeli, A.; Crystal, R.G.; Oppenheim, F.G.; Baum, B.J. Transfer of a gene encoding the anticandidal protein histatin 3 to salivary glands. Hum. Gene Ther. 1996, 7, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Isenman, L.D.; Rothman, S.S. Transport of alpha-amylase across the basolateral membrane of the pancreatic acinar cell. Proc. Natl. Acad. Sci. USA. 1977, 74, 4068–4072. [Google Scholar] [CrossRef] [PubMed]
- Rosa, V.; Della Bona, A.; Cavalcanti, B.N.; Nör, J.E. Tissue engineering: From research to dental clinics. Dent. Mater. 2012, 28, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Zheng, L. Gene therapy for dentin regeneration with bone morphogenetic proteins. Curr. Gene Ther. 2006, 6, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kiso, H.; Saito, K.; Togo, Y.; Tsukamoto, H.; Huang, B.; Besho, K. Feasibility of Gene Therapy for Tooth Regeneration by Stimulation of a Third Dentition; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Chen, Y.; Chen, P.K.; Jeng, L.; Huang, C.; Yang, L.; Chung, H.; Chang, S.C. Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: An alternative to alveolaplasty. Gene Ther. 2008, 15, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Bluteau, G.; Luder, H.; De Bari, C.; Mitsiadis, T. Stem cells for tooth engineering. Eur. Cell. Mater. 2008, 16, 1–9. [Google Scholar] [PubMed]
- Monteiro, N.; Yelick, P.C. Advances and perspectives in tooth tissue engineering. J. Tissue Eng. Regen. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Yao, Y.; Wei, D.; Wang, R.; Wu, Q. A controlled double-duration inducible gene expression system for cartilage tissue engineering. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Pofali, P.; Park, T.; Singh, B.; Cho, K.; Maharjan, S.; Dandekar, P.; Jain, R.; Choi, Y.; Arote, R. Gene therapy for bone tissue engineering. Tissue Eng. Regener. Med. 2016, 13, 111–125. [Google Scholar] [CrossRef]




| Year | Key Advancements | Ref. |
|---|---|---|
| 1928 | Griffith′s experiments with pneumococcal bacteria and introducing transforming principles | [17] |
| 1944 | Purification of transforming substance; first reported deoxyribonucleic acid (DNA) causes the transformation | [18] |
| 1952 | Transduction (transfer of genetic through bacteria) was introduced for the first time | [19] |
| 1953 | Double helix structure of DNA was described | [20] |
| 1961 | It was reported that viral infections can inherit genetic mutations | [21] |
| 1962 | First ever DNA-mediated heritable transformation of a biochemical trait | [22] |
| 1973 | First gene therapy trial conducted in humans | [23] |
| 1989 | Reported gene transfer in humans | [24] |
| 1990 | FDA approved gene therapy trial in humans for therapeutic applications. | [2] |
| 1995 | Gene therapy introduced for dental applications | [4] |
| 1999 | Jesse Gelsinger died during a clinical trial of gene therapy | [25] |
| 2003 | China approved gene therapy for clinical applications | [2] |
| 2005 | Gendicine™ (an adenoviral vector) approved for the treatment of squamous cell carcinoma | [26,27] |
| 2009 | Cerepro® (an adenoviral vector) gene therapy for the treatment of malignant brain tumors | [28] |
| 2012 | A gene therapy product (Glybera) that is an adeno-associated viral vector was recommended for the European Union | [2] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, N.; Raza, H.; Ahmed, S.; Khurshid, Z.; Zafar, M.S. Gene Therapy: A Paradigm Shift in Dentistry. Genes 2016, 7, 98. https://doi.org/10.3390/genes7110098
Siddique N, Raza H, Ahmed S, Khurshid Z, Zafar MS. Gene Therapy: A Paradigm Shift in Dentistry. Genes. 2016; 7(11):98. https://doi.org/10.3390/genes7110098
Chicago/Turabian StyleSiddique, Nida, Hira Raza, Sehrish Ahmed, Zohaib Khurshid, and Muhammad Sohail Zafar. 2016. "Gene Therapy: A Paradigm Shift in Dentistry" Genes 7, no. 11: 98. https://doi.org/10.3390/genes7110098
APA StyleSiddique, N., Raza, H., Ahmed, S., Khurshid, Z., & Zafar, M. S. (2016). Gene Therapy: A Paradigm Shift in Dentistry. Genes, 7(11), 98. https://doi.org/10.3390/genes7110098