3.2. Fog Chemistry
A total of 293 fog samples was collected between 14 January 2011 08:00 p.m. and 31 January 2011 08:00 a.m. Most of the samples (83%) covered a collection time of 1 h, 14% were collected over 2 h, while only 10 samples extended over longer collection time periods of up to 5 h (1 sample).
Figure 1 (second panel) shows that there is a very large variability of the pH during the 18-days experimental period, although there is no apparent or large change in wind direction. Only during the time period of the shift of the wind direction on 23 January, one fog sample was collected between 02:00 a.m. and 03:00 a.m. during winds from the south. This sample is exceptional in the sense that it exhibits the lowest of all pHs (3.17) and high concentrations of ions, specifically sodium, chloride, and sulfate (440 µeq.·L
−1 NH
4+; 1000 µeq.·L
−1 Na
+; 22 µeq.·L
−1 K
+; 60 µeq.·L
−1 Ca
2+; 150 µeq.·L
−1 Mg
2+; 1900 µeq.·L
−1 SO
42−; 310 µeq.·L
−1 NO
3−; 1200 µeq.·L
−1 Cl
−). This sample is apparently influenced by the local volcanic activity of the Yangmingshan National Park.
Panel 3 in
Figure 1 shows the nss-SO
42− concentrations in fog water. Note that the scale is logarithmic and that it exhibits strikingly similar features as the pH scale of fog in the panel above. The LWC (panel 4) exhibits a much lower variability than the concentration. The ion load (panel 5), which is the computed product of the concentration and the LWC, varies as largely as the concentration. It is therefore the concentration in fog water that drives the ion load much more than it is the LWC. The situation is similar to that of all ions (not shown). Based on these results, we will, in the remainder of this paper, analyze the concentrations of ions in fog water bearing in mind that they are the main drivers for the ion load as well.
Figure 1.
Time series of meteorological and air and rain chemistry data at Mt. Bamboo, northern Taiwan from 15 to 30 January 2011. Top panel: Wind direction as measured at the collection site (filled circles) and as derived from the backward trajectories (open circles); 2nd panel: pHs in fog (full circles) and in rain (open squares) at Mt. Bamboo; 3rd panel: nss-SO42− concentration in fog water; 4th panel: LWC in air (left axis) and cumulative rain (right axis); 5th panel: nss-SO42− load in air; bottom panel: NOx mixing ratio and PM10 concentrations at the TEPA site.
Figure 1.
Time series of meteorological and air and rain chemistry data at Mt. Bamboo, northern Taiwan from 15 to 30 January 2011. Top panel: Wind direction as measured at the collection site (filled circles) and as derived from the backward trajectories (open circles); 2nd panel: pHs in fog (full circles) and in rain (open squares) at Mt. Bamboo; 3rd panel: nss-SO42− concentration in fog water; 4th panel: LWC in air (left axis) and cumulative rain (right axis); 5th panel: nss-SO42− load in air; bottom panel: NOx mixing ratio and PM10 concentrations at the TEPA site.
For further analysis, the World Meteorological Organization (WMO) quality criteria for precipitation water [
19] were applied to examine the quality of the chemical analysis of the fog samples. Of the 293 samples, 233 complied with the ion balance (ratio of total analyzed anions’ load
versus total analyzed cations’ load) data quality objective (DQO), 258 samples complied with the conductivity DQO (calculated
versus measured conductivity), and 203 samples complied with both DQOs. The majority (72%) of the ion balances of the samples that did not comply with the DQOs were positive, which indicates that organic acids such as acetic or formic acids played a role but were not measured in our routine. For the other samples, there is no indication of why the DQOs were not met. In order to avoid the rejection of any sample that may yield useful information, but also to comply with the quality assurance and quality control (QAQC) guidelines of the WMO, we will analyze the data in two subsets, one subset with samples that met all DQOs (QA = 1), and the other (QA = 2) with samples that did not meet one DQO or both.
Figure 2 presents the relationship of some ions in relation to the sample pHs as scatterplots. The
p-notation is applied in order to illustrate the similarity of the concentration patterns. These suggest that NH
4+, SO
42−, and NO
3− covary very much with H
+ and also with each other (not shown). It has been shown for many locations that the ions NH
4+, SO
42−, and NO
3− make up a large fraction of the ion balance, typically 85% or more (e.g., [
5,
7,
8,
20,
21]). In our data set, the maximum contribution of NH
4+, SO
42−, and NO
3− is 66%, mainly because our site is influenced by the ocean and the sea-salt-derived ions Na
+ and Cl
− contribute more to the ion balance. This phenomenon has been described for other sites as well (e.g., [
12,
22]). Nevertheless, it is the balance between the three ions NH
4+, SO
42−, and NO
3− that determines the pH of the fog samples. Calcium plays a role as well, which will be discussed below. Ammonium and NO
3− apparently show steeper correlations than the respective 1:1 lines (
Figure 2). Sulfate correlated well with H
+.
There are six outliers in
Figure 2 which can be best recognized in the upper-left panel with pH > 4 and pNO
3− < 4. These six samples also stand out in
Figure 1 on 30 January between 11:00 a.m. and 06:00 p.m. because they clearly fall out from the temporal series of pH (second panel from top), but not in the nss-SO
42− (3rd panel) and most of the other ion concentrations (not shown). In
Figure 2, these samples are least prominent in the pCa
2+-plot (lower-right). There was a period of enhanced Ca
2+-concentrations in fog between 02:00 a.m. and 01:00 p.m. on 30 January.
Figure 1 also shows (bottom panel) that there was a period with enhanced PM
10 concentrations from about 01:00 p.m. on 29 January until the end of the experimental period, peaking with 52 µg·m
−3 at 07:00 a.m. on 30 January and indicating the advection of dust through long-range transport. Although the respective six fog samples do not meet either DQO (QA = 2), and although the periods of high pH do not match perfectly with the periods of enhanced PM
10, we presume that a real phenomenon was identified. The respective air masses likely picked up some Ca
2+-rich particles in the desert region of northern China and Mongolia 96 to 48 h before arrival at Mt. Bamboo (as analyzed from the individual backward trajectories, not shown in detail), but also incorporated sulfur-rich emissions from the industrial regions of eastern China during the two days before its arrival at Mt. Bamboo. Although the pHs of these samples are normal in our data set, they seem to be partly controlled by the presence of calcium.
Another six samples are rather prominent in
Figure 2 through their pHs above 5.6. Five of these six samples were collected in series on 28 January between 03:00 a.m. and 08:00 a.m. Only two can be detected in
Figure 1 (second panel from above) because the other three are out of the displayed scale (pHs of 6.1, 6.3, 6.4). These samples are very clean, with total ion concentrations (neglecting the sea salt components Na
+ and Cl
−) between 11 and 21 µeq.·L
−1. The median Na
+ and Cl
−-concentrations are 33 and 37 µeq.·L
−1, respectively, reflecting the strong influence of sea salt. Only two of the five samples meet the DQO of the WMO. The sixth sample with very high pH was collected at 08:00 p.m. on 14 January. It is a QA = 2 sample with high concentrations of Na
+, Cl
− and other ions. The high pH is, in this case, caused by the neutralization of the acidity from SO
42− through high Ca
2+-concentrations. The backward trajectory (not shown in detail) confirms that the respective air mass had travelled over the desert areas in NW China, then over the industrialized areas of eastern China towards Shandong province, and eventually two more days at low altitude over the East China Sea before arriving at Mt. Bamboo.
Figure 2.
Scatterplots of nitrate (upper left panel), non-sea-salt sulfate (upper right panel), ammonium (lower left panel), and calcium (lower right panel) against pH in fog, and respective 1:1 lines. The p-notation represents the –log10 of the respective concentration in unit eq.·L−1, analogous to the definition of pH. Filled symbols are used for samples that met the DQO criteria of WMO (QA = 1), open circles for QA = 2 samples.
Figure 2.
Scatterplots of nitrate (upper left panel), non-sea-salt sulfate (upper right panel), ammonium (lower left panel), and calcium (lower right panel) against pH in fog, and respective 1:1 lines. The p-notation represents the –log10 of the respective concentration in unit eq.·L−1, analogous to the definition of pH. Filled symbols are used for samples that met the DQO criteria of WMO (QA = 1), open circles for QA = 2 samples.
The upper-right plot in
Figure 2 further indicates that there are two main groups of data with high or low concentrations of nss-SO
42−, the groups appear to be rather separate from each other. We further studied this feature in
Figure 3. We plotted histograms for pH, pNH
4+, pnss-SO
42−, pNO
3−, pCa
2+, and pNa
+, for QA = 1 data (filled bars) and QA = 2 data (open bars). All ion concentration histograms support the hypothesis that two or more groups of data exist, although the separation of the groups is most evident for pnss-SO
42−.
Figure 3.
Histograms of the counting statistics of pH, pNH4+, pnss-SO42−, pNO3−, pCa2+, and pNa+ data in fog. Filled bars indicate the samples in full compliance with the DQOs (QA = 1 data), open bars show the data not in compliance (QA = 2). The lines in the left-middle panel indicates a two-mode Gaussian distribution approximating the full data set (QA = 1 plus QA = 2 data subsets), for illustration only.
Figure 3.
Histograms of the counting statistics of pH, pNH4+, pnss-SO42−, pNO3−, pCa2+, and pNa+ data in fog. Filled bars indicate the samples in full compliance with the DQOs (QA = 1 data), open bars show the data not in compliance (QA = 2). The lines in the left-middle panel indicates a two-mode Gaussian distribution approximating the full data set (QA = 1 plus QA = 2 data subsets), for illustration only.
Based on this finding, we separated the data pool into two groups for further analysis, group_1 with pnss-SO
42− < 3.52 (nss-SO
42− > 300 µeq.·L
−1), and group_2 for p nss-SO
42− > 3.70 (nss-SO
42− < 200 µeq.·L
−1). Between these groups were two samples that did not meet the DOQs. They are excluded from the following “group analysis” in order to achieve a better separation of the two groups. The chemical information (medians and interquartile ranges) of the two groups are presented in
Table 1. The full data set is presented separately from the data that comply with the DQOs (QA = 1) so that there are four columns representing the two groups.
The concentrations of all ions are much higher in group_1 than in group_2. This is not surprising, since the grouping was done on the basis of the nss-SO
42− data and the concentrations covary to a high degree (
Figure 2). The concentration ratios of the medians between the groups are between 30 (Mg
2+) and 150 (NH
4+) for QA = 1 data and between 8.0 and 130 for the same ions and for the entire data set (QA = 1 data and QA = 2 data). The respective difference in pH (just below 1.3 units) reflects a variability between the groups that is at the lower end of the ion variabilities (just below a factor of 20 for the re-calculated H
+-concentrations).
Table 1.
pH and concentrations of ions (medians and interquartile ranges) for 2 groups of samples (group_1 for high nss-SO42− data, group_2 for cleaner samples), presented separately for quality assurance (QA) = 1 samples, and for all data (QA = 1 and QA = 2). Anbu rain data, as collected daily, are also shown.
Table 1.
pH and concentrations of ions (medians and interquartile ranges) for 2 groups of samples (group_1 for high nss-SO42− data, group_2 for cleaner samples), presented separately for quality assurance (QA) = 1 samples, and for all data (QA = 1 and QA = 2). Anbu rain data, as collected daily, are also shown.
| -- | -- | Fog QA = 1 and QA = 2 Data | Fog QA = 1 and QA = 2 Data | Fog QA = 1 Data | Fog QA = 1 Data | Rain |
|---|
| Group_1 | Group_2 | Group_1 | Group_2 |
|---|
| n = 49 | n = 242 | n = 20 | n = 183 | n = 16 |
|---|
| pH | 25 percentile | 3.46 | 4.29 | 3.49 | 4.39 | 4.15 |
| median | 3.52 | 4.80 | 3.54 | 4.81 | 4.86 |
| 75 percentile | 3.65 | 5.17 | 3.66 | 5.06 | 5.91 |
| nss-SO42− | 25 percentile | 500 | 5.6 | 570 | 5.6 | 12 |
| median | 770 | 11 | 700 | 11 | 30 |
| 75 percentile | 930 | 34 | 830 | 32 | 92 |
| NO3− | 25 percentile | 240 | 2.8 | 290 | 2.7 | 4.7 |
| median | 410 | 5.2 | 420 | 5.0 | 14 |
| 75 percentile | 640 | 18 | 690 | 15 | 61 |
| NH4+ | 25 percentile | 180 | 1.4 | 230 | 1.5 | 3.9 |
| median | 440 | 3.5 | 470 | 3.0 | 9.0 |
| 75 percentile | 630 | 11 | 600 | 9.7 | 60 |
| Ca2+ | 25 percentile | 23 | 0.9 | 76 | 0.9 | 12 |
| median | 67 | 2.4 | 140 | 2.1 | 35 |
| 75 percentile | 140 | 9.7 | 240 | 6.2 | 66 |
| Na+ | 25 percentile | 550 | 22 | 1500 | 22 | 40 |
| median | 1000 | 57 | 2100 | 50 | 160 |
| 75 percentile | 1800 | 160 | 2800 | 110 | 290 |
| K+ | 25 percentile | 17 | 0.4 | 31 | 0.4 | 1.3 |
| median | 32 | 1.2 | 40 | 1.0 | 4.8 |
| 75 percentile | 51 | 4.4 | 68 | 2.8 | 9.1 |
| Mg2+ | 25 percentile | 40 | 3.3 | 140 | 3.5 | 8.3 |
| median | 86 | 11 | 290 | 9.6 | 36 |
| 75 percentile | 240 | 36 | 470 | 25 | 69 |
| Cl− | 25 percentile | 650 | 25 | 1500 | 25 | 45 |
| median | 990 | 60 | 2100 | 54 | 170 |
| 75 percentile | 19000 | 180 | 2700 | 110 | 280 |
| NO3−/nss-SO42− | 25 percentile | 0.46 | 0.34 | 0.48 | 0.34 | 0.38 |
| median | 0.68 | 0.54 | 0.67 | 0.51 | 0.58 |
| 75 percentile | 0.78 | 0.75 | 0.87 | 0.70 | 0.83 |
| Cl−/Na+ | 25 percentile | 0.95 | 1.00 | 0.95 | 1.02 | 0.97 |
| median | 1.11 | 1.09 | 1.02 | 1.11 | 1.06 |
| 75 percentile | 1.35 | 1.17 | 1.06 | 1.17 | 1.11 |
Group_2 fog sample represent very clean fog water. With exception of the sea-salt derived ions (Na
+, Cl
−, Mg
2+), the concentrations are by a factor of about 10 lower than the fog concentrations from Mt. Lulin in Central Taiwan at 2862 m a.s.l. [
23]. The 25 percentiles of concentrations are in the order of the cleanest fog water reported in the literature (e.g., [
12,
24]). The NO
3−/nss-SO
42− ratio of the medians is 0.43 for QA = 1 data and 0.47 for the entire data set. The median pHs are 4.94 or 4.82, respectively, which is representative for rather pristine pH of the atmospheric liquid phase [
25]. The 75 percentiles are well above pH 5. In contrast, group_1 samples represent rather high concentrations of ions in fog. The median NO
3−/nss-SO
42− ratio is 0.54 for the full data set and 0.47 for the QA = 1 data. The biggest enhancement of concentrations in group_1 as compared to group_2 was found for NH
4+.
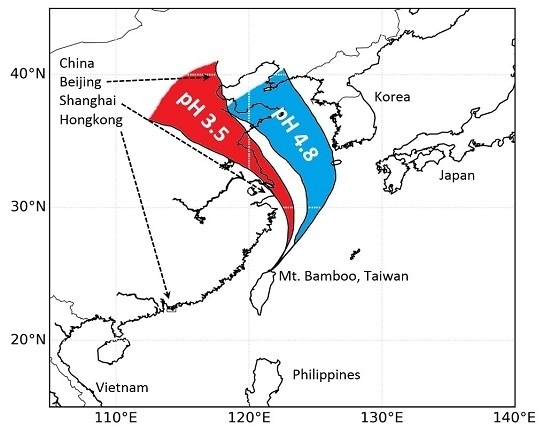
Ensemble backward trajectories for group_1 and group_2 fog samples are shown as medians and interquartile ranges in
Figure 4. All trajectories arrive Taiwan from the northeast. Group_1 trajectories originate from the regions around the greater Beijing area, the Shandong province, Nanjing and Shanghai regions. In contrast, the group_2 trajectories travelled much more time over the South China Sea. The terrestrial influence is from the northeast of China, Manchuria province and considerable less influence from the Beijing region. It is evident from this analysis why group_2 samples are considerably less polluted than group_1 fog, which is strongly influenced by the heavily populated and industrialized regions of eastern China.
Figure 4.
Map section of East Asia with ensemble 48-h back-trajectories arriving at Mt. Bamboo, Taiwan, during the experimental period in January 2011. The red-color trajectory sector represents the 25 to 75 percentiles of group_1 (more polluted) fog samples (all data, n = 49), group_2 (blue color) are the respective trajectories for less polluted samples (n = 242).
Figure 4.
Map section of East Asia with ensemble 48-h back-trajectories arriving at Mt. Bamboo, Taiwan, during the experimental period in January 2011. The red-color trajectory sector represents the 25 to 75 percentiles of group_1 (more polluted) fog samples (all data, n = 49), group_2 (blue color) are the respective trajectories for less polluted samples (n = 242).
The NO
3−/nss-SO
42− ratios of our fog samples were within a narrow range between 0.34 and 0.87 for the interquartile ranges (
Table 1). The median for the group_2 samples was about 0.53. In the group_1 data set, the median ratio is 0.67, which shows that the nitrate plays a slightly more important role than in group_2. This data is in general agreement with data from coastal and mountain sites in the USA [
22] and a mountain site in China [
7]. Another mountain site in Japan, which is, however, influenced by advection of highly polluted air masses, exhibits much higher ratios, throughout above unity [
20].
3.3. Rain Chemistry and Scavenging
Two data subsets are available for the rain chemistry. One covers the samples taken daily from 15 January through 31 January 2011. On 23 January, no sample was taken for chemical analysis due to a lack of sufficient sample volume, so that a total of 16 samples are available. This daily data (
Table 1, last column) does not reflect the same variability as the fog samples (
Table 1), which had been taken on an hourly basis. The additional recording of rain pH on an hourly basis (open symbols in
Figure 1, second panel), however, indicates that the variability of the composition of the rain is as large as that of the fog. The pHs are similar, although there seems to be a temporal tendency of higher pHs in rain as compared to fog, particularly on 21–22 January and on 29–30 January.
All 16 rain samples do not meet the ion balance data quality objective of the WMO (the conductivity DQO could not be tested due to the absence of measured conductivity data). The rain sample ion charges were positive, with cation surpluses between 31% and 40%. This indicates a systematic error with an important anion not having been analyzed. Likely, one or more carboxylic acids were present in the rainwater but not analyzed or other organic species such as organosulfates may have been formed during atmospheric transport and not analyzed, too [
26]. Although the DQO was not met, we will have a closer look at the chemical composition of the rain samples in the following section.
We analyze the below-cloud scavenging by applying the concept described in detail by Lin and Peng [
13] for Taipei rain. Briefly, it is assumed that the differences in concentrations in rain from those in the overlaying cloud (fog at the higher mountain site) is caused (i) by dilution of the cloud water and (ii) by scavenging of aerosols by raindrops falling through the air mass between the cloud base and the surface. The dilution is quantified through the decrease of the Na
+ concentration, assuming that the Na
+ in both cloud water and rainwater originated exclusively from sea salt and no scavenging of sea-salt-containing aerosol particles took place. Based on the comparison of the Na
+ concentrations in cloud water and fog water, additional changes (increases) of concentrations of other ions are attributed to below-cloud scavenging. The contribution of below-cloud scavenging f is quantified for each event and each ion. For example, a value f(NO
3−) of 60% would indicate that 60% of the nitrate in rain originates from below-cloud scavenging, while the remaining 40% were originally present in the cloud water.
Table 2 shows the quartiles of
f-values for ions in the 16 rain events. Chloride, NO
3−, nss-SO
42−, and K
+ exhibit no contribution of below-cloud scavenging to the rainwater chemistry. In fact,
f-values below zero are physically meaningless and thus interpreted here as zero,
i.e., no contribution of below-cloud scavenging took place. Likewise positive
f-values below 35% are considered to be zero rather than indicators for below-cloud scavenging. Ammonium (f(NH
4+) up to 54%) and Mg
2+ (f(Mg
2+) up to 67%) exhibit some contributions in 5 and 3 rain samples, respectively (data not shown in detail). Most striking are the contributions of below-cloud scavenging of calcium. The f(Ca
2+)-values are always above 25% and reach up to 92%. It seems that a contribution of Ca
2+-rich soil and bedrock material from the surrounding of the sampling sites [
27] contributes largely to the respective Ca
2+-concentration in rainwater.
Figure 5 shows a scatterplot of f(Ca
2+)
versus ΔpH, which is the difference between rain-pH and cloud water pH: ΔpH = pH
rain − pH
fog. Whenever the rain pH was 0.5 or more units above the cloud water pH, f(Ca
2+) was above 0.5. Clearly the scavenging of Ca
2+-containing particles increased the pH of rain in these cases.
Table 2.
Quartiles of contributions of below-cloud scavenging (f-values) for ions at the Anbu site from 14 through 31 January 2011.
Table 2.
Quartiles of contributions of below-cloud scavenging (f-values) for ions at the Anbu site from 14 through 31 January 2011.
| -- | 25 Percentile | Median | 75 Percentile |
|---|
| f(Cl−) | −0.08 | 0.00 | 0.10 |
| f(NO3−) | −0.06 | 0.05 | 0.20 |
| f(nss-SO42−) | −0.25 | 0.10 | 0.34 |
| f(NH4+) | −0.09 | 0.25 | 0.41 |
| f(Na+) | 0.00 | 0.00 | 0.00 |
| f(K+) | 0.06 | 0.30 | 0.40 |
| f(Mg2+) | 0.00 | 0.09 | 0.15 |
| f(Ca2+) | 0.49 | 0.82 | 0.88 |
Figure 5.
Scatterplot of the contribution of below-cloud scavenging to the Ca2+-concentration in rainwater f(Ca2+), against the difference of rainwater pH at the TEPA-site Anbu minus cloud water pH, based on daily rainwater samples and volume-weighted 24-h fog water concentrations between 14 and 31 January 2011.
Figure 5.
Scatterplot of the contribution of below-cloud scavenging to the Ca2+-concentration in rainwater f(Ca2+), against the difference of rainwater pH at the TEPA-site Anbu minus cloud water pH, based on daily rainwater samples and volume-weighted 24-h fog water concentrations between 14 and 31 January 2011.