The Fate of Dissolved Organic Matter (DOM) During Bank Filtration under Different Environmental Conditions: Batch and Column Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Batch Experiments
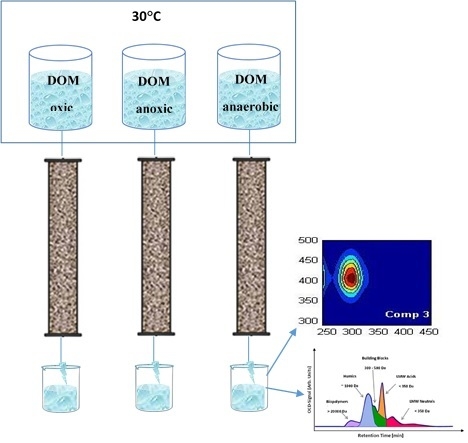
2.2. Column Experiments
2.3. Analytical Methods
2.4. PARAFAC Modelling
2.5. Statistical Analysis
3. Results
3.1. PARAFAC Components
3.2. Batch Experiments
3.2.1. Characteristics of Influent Water DOM
3.2.2. Bulk Organic Matter Parameters
3.2.3. LC-OCD Analysis
3.2.4. PARAFAC-EEM Analysis
3.3. Column Experiments
3.3.1. Characteristics of Influent Water DOM
3.3.2. Bulk Organic Matter Parameters
3.3.3. LC-OCD Analysis
3.3.4. PARAFAC-EEM Analysis
4. Discussion
4.1. Impact of Temperature and Influent Organic Composition on DOM Behaviour
4.2. Impact of Redox Conditions on DOM Behaviour
5. Conclusions
- A positive correlation was found between DOM biodegradation and raw water concentration, which was likely due to the higher microbial activity associated with sand, as determined by ATP measurements of the biomass attached to the sand grains.
- The removal of DOM during filtration is significantly impacted by temperature variation, with higher removal at lower temperatures.
- LC-OCD results revealed that the labile compounds (i.e., biopolymers) are highly removed (>80%) under oxic filtration, regardless of the temperature and organic matter composition of the feed water. Likewise, the PARAFAC protein-like component exhibited the highest reduction at all temperatures studied.
- Humic compound removal exhibited a significant dependence on temperature, with higher removal at a lower temperature. PARAFAC analysis indicated that terrestrial humic components are the least persistent humic type adsorbed at a lower temperature. The contradictory behaviour of protein and humic compounds explains the positive relationship between SUVA and temperature.
- DOM was preferentially removed under oxic conditions; its removal decreased by 5–10% under anoxic, and by 7–15% under anaerobic conditions. LC-OCD results reveal that biopolymers are the most impacted fraction by altering the redox conditions. Humic compounds also exhibited a lower removal efficiency (with less extent) under sub-oxic conditions. Therefore, post-treatment steps should be considered in case of sub-oxic filtration.
- In general, this study revealed that the BF removal efficiency for DOM components under arid conditions (high temperature) is determined by the feed water organic composition and redox conditions in the infiltration area.
- Finally, this study shows that PARAFAC-EEM and LC-OCD can be promising tools to provide further insight into BF processes and for determining the treatment efficiency for DOM components.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stahlschmidt, M.; Regnery, J.; Campbell, A.; Drewes, J. Application of 3D-fluorescence/PARAFAC to monitor the performance of managed aquifer recharge facilities. J. Water Reuse Desalin. 2015, 6, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Hiscock, K.M.; Grischek, T. Attenuation of groundwater pollution by bank filtration. J. Hydrol. 2002, 266, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Tufenkji, N.; Ryan, J.N.; Elimelech, M. The Promise of Bank Filtration. Environ. Sci. Technol. 2002, 36, 422A–428A. [Google Scholar] [CrossRef] [PubMed]
- Bartak, R.; Grischek, T.; Ghodeif, K.; Wahaab, R. Shortcomings of the RBF Pilot Site in Dishna, Egypt. J. Hydrol. Eng. 2014. [Google Scholar] [CrossRef]
- Boving, T.B.; Choudri, B.S.; Cady, P.; Cording, A.; Patil, K.; Reddy, V. Hydraulic and Hydrogeochemical Characteristics of a Riverbank Filtration Site in Rural India. Water Environ. Res. 2014, 86, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Melin, G.; Linsky, R.B. Riverbank Filtratio: Improving Source-Water Quality; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; NWRI, National Water Research Institute: Fountain Valley, CA, USA, 2002. [Google Scholar]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.-N.; Amy, G. Differentiation of wastewater effluent organic matter (EfOM) from natural organic matter (NOM) using multiple analytical techniques. Water Science Technol. 2008, 57, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xian, Q.; Lu, J.; Gong, T.; Li, A.; Feng, J. Evaluation of DBPs formation from SMPs exposed to chlorine, chloramine and ozone. J. Water Health 2016, 15, 185–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghoth, S.A.; Sharma, S.K.; Amy, G.L. Tracking natural organic matter (NOM) in a drinking water treatment plant using fluorescence excitation-emission matrices and PARAFAC. Water Res. 2011, 45, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yates, S.R. Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: A review. Sci. Total Environ. 2018, 640, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.A.; Kulkarni, H.V.; Mladenov, N.; Johannesson, K.; Hettiarachchi, G.M.; Bhattacharya, P.; Kumar, N.; Weeks, J.; Galkaduwa, M.; Datta, S. Biogeochemical Controls on the Release and Accumulation of Mn and As in Shallow Aquifers, West Bengal, India. Front. Environ. Sci. 2017, 5, 29. [Google Scholar] [CrossRef]
- Chianese, S.; Iovino, P.; Leone, V.; Musmarra, D.; Prisciandaro, M. Photodegradation of Diclofenac Sodium Salt in Water Solution: Effect of HA, NO3− and TiO2 on Photolysis Performance. Water Air Soil Pollut. 2017, 228, 270. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Evaluation of soluble microbial products (SMP) on membrane fouling in membrane bioreactors (MBRs) at the fractional and overall level: A review. Rev. Environ. Sci. Bio/Technol. 2018, 17, 71–85. [Google Scholar] [CrossRef]
- Rostad, C.E.; Leenheer, J.A.; Katz, B.; Martin, B.S.; Noyes, T.I. Characterization and Disinfection By-Product Formation Potential of Natural Organic Matter in Surface and Ground Waters from Northern Florida. ACS Symp. Ser. 2000, 761, 154–172. [Google Scholar] [CrossRef]
- Drewes, J.E.; Summers, R.S. Natural Organic Matter Removal During Riverbank Filtration: Current Knowledge and Research Needs. In Riverbank Filtration: Improving Source-Water Quality; Ray, C., Melin, G., Linsky, R.B., Eds.; Springer: Dordrecht, The Nerthands, 2003; pp. 303–309. [Google Scholar]
- Gross-Wittke, A.; Gunkel, G.; Hoffmann, A. Temperature effects on bank filtration: Redox conditions and physical-chemical parameters of pore water at Lake Tegel, Berlin, Germany. J. Water Clim. Chang. 2010, 1, 55–66. [Google Scholar] [CrossRef]
- Pan, W.; Huang, Q.; Huang, G. Nitrogen and Organics Removal during Riverbank Filtration along a Reclaimed Water Restored River in Beijing, China. Water 2018, 10, 491. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Magic-Knezev, A.; Amy, G. Fate of effluent organic matter (EfOM) and natural organic matter (NOM) through riverbank filtration. Water Sci. Technol. 2008, 57, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; von Rohr, M.R.; Hering, J.G.; Kohler, H.-P.E.; Schirmer, M.; von Gunten, U. NOM degradation during river infiltration: Effects of the climate variables temperature and discharge. Water Res. 2013, 47, 6585–6595. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, E.; Scholtis, A. Exchange between a river and groundwater, assessed with hydrochemical data. Hydrol. Earth Syst. Sci. 2011, 15, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Derx, J.; Andreas, H.F.; Matthias, Z.; Liping, P.; Jack, S.; Blaschke, A.P. Evaluating the effect of temperature induced water viscosity and density fl uctuations on virus and DOC removal during river bank fi ltration—A scenario analysis. River Syst. 2012, 20, 169–183. [Google Scholar] [CrossRef]
- Maeng, S.K.; Ameda, E.; Sharma, S.K.; Grützmacher, G.; Amy, G.L. Organic micropollutant removal from wastewater effluent-impacted drinking water sources during bank filtration and artificial recharge. Water Res. 2010, 44, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Ghodeif, K.; Grischek, T.; Bartak, R.; Wahaab, R.; Herlitzius, J. Potential of river bank filtration (RBF) in Egypt. Environ. Earth Sci. 2016, 75, 671. [Google Scholar] [CrossRef]
- Sprenger, C.; Lorenzen, G.; Asaf, P. Environmental Tracer Application and Purification Capacity at a Riverbank Filtration Well in Delhi (India). J. Indian Water Works Assoc. 2012, 1, 25–32. [Google Scholar]
- Misra, A.K. Climate change and challenges of water and food security. Int. J. Sustain. Built Environ. 2014, 3, 153–165. [Google Scholar] [CrossRef]
- Guigue, J.; Mathieu, O.; Lévêque, J.; Mounier, S.; Laffont, R.; Maron, P.A.; Navarro, N.; Chateau, C.; Amiotte-Suchet, P.; Lucas, Y. A comparison of extraction procedures for water-extractable organic matter in soils. Eur. J. Soil Sci. 2014, 65, 520–530. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Abel, C.; Magic-Knezev, A.; Amy, G.L. Role of biodegradation in the removal of pharmaceutically active compounds with different bulk organic matter characteristics through managed aquifer recharge: Batch and column studies. Water Res. 2011, 45, 4722–4736. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Snyder, S.A. Sample handling and data processing for fluorescent excitation-emission matrix (EEM) of dissolved organic matter (DOM). Chemosphere 2018, 193, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Chen, J.P.; Hung, Y.T.; Shammas, N.K. Membrane and Desalination Technologies; Humana Press: New York, NY, USA, 2008. [Google Scholar]
- Abushaban, A.; Mangal, M.; Salinas-Rodriguez, S.G.; Nnebuo, C.; Mondal, S.; Goueli, S.; Schippers, J.; Kennedy, M. Direct Measurement of ATP in Seawater and Application of ATP to Monitor Bacterial Growth Potential in SWRO Pre-Treatment Systems. Desalin. Water Treat. 2017, 99, 91–101. [Google Scholar] [CrossRef]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography-Organic carbon detection-Organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.M.; Bro, R. Practical aspects of PARAFAC modeling of fluorescence excitation-emission data. J. Chemometr. 2003, 17, 200–215. [Google Scholar] [CrossRef] [Green Version]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Colin, A.S.; Rasmus, B. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef] [Green Version]
- Cuss, C.W.; Guéguen, C.; Andersson, P.; Porcelli, D.; Maximov, T.; Kutscher, L. Advanced Residuals Analysis for Determining the Number of PARAFAC Components in Dissolved Organic Matter. Appl. Spectrosc. 2016, 70, 334–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-T.; Chen, S.-Y.; Xu, Z.-X.; Li, Y.; Shuang, C.-D.; Li, A.-M. Characterization of Dissolved Organic Matter in Municipal Wastewater Using Fluorescence PARAFAC Analysis and Chromatography Multi-Excitation/Emission Scan: A Comparative Study. Environ. Sci. Technol. 2014, 48, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.A.; Amon, R.M.W.; Stedmon, C.; Duan, S.; Louchouarn, P. The use of PARAFAC modeling to trace terrestrial dissolved organic matter and fingerprint water masses in coastal Canadian Arctic surface waters. J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Osburn, C.L.; Handsel, L.T.; Peierls, B.L.; Paerl, H.W. Predicting Sources of Dissolved Organic Nitrogen to an Estuary from an Agro-Urban Coastal Watershed. Environ. Sci. Technol. 2016, 50, 8473–8484. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Inamdar, S.; Scott, D. Comparison of Two PARAFAC Models of Dissolved Organic Matter Fluorescence for a Mid-Atlantic Forested Watershed in the USA. J. Ecosyst. 2013, 2013, 16. [Google Scholar] [CrossRef]
- Kulkarni, H.V.; Mladenov, N.; Johannesson, K.H.; Datta, S. Contrasting dissolved organic matter quality in groundwater in Holocene and Pleistocene aquifers and implications for influencing arsenic mobility. Appl. Geochem. 2017, 77, 194–205. [Google Scholar] [CrossRef]
- Rehman, Z.; Jeong, S.; Tabatabai, S.; Emwas, A.; Leiknes, T. Advanced characterization of dissolved organic matter released by bloom-forming marine algae. Desalin. Water Treat. 2017, 69, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sharp, J.O.; Saikaly, P.E.; Ali, S.; Alidina, M.; Alarawi, M.S.; Keller, S.; Hoppe-Jones, C.; Drewes, J.E. Dissolved Organic Carbon Influences Microbial Community Composition and Diversity in Managed Aquifer Recharge Systems. Appl. Environ. Microbiol. 2012, 78, 6819. [Google Scholar] [CrossRef] [PubMed]
- Massmann, G.; Greskowiak, J.; Dünnbier, U.; Zuehlke, S.; Knappe, A.; Pekdeger, A. The impact of variable temperatures on the redox conditions and the behaviour of pharmaceutical residues during artificial recharge. J. Hydrol. 2006, 328, 141–156. [Google Scholar] [CrossRef]
- Abel, C.; Sharma, S.K.; Malolo, Y.N.; Maeng, S.K.; Kennedy, M.D.; Amy, G.L. Attenuation of Bulk Organic Matter, Nutrients (N and P), and Pathogen Indicators During Soil Passage: Effect of Temperature and Redox Conditions in Simulated Soil Aquifer Treatment (SAT). Water Air Soil Pollut. 2012, 223, 5205–5220. [Google Scholar] [CrossRef]
- Alidina, M.; Shewchuk, J.; Drewes, J.E. Effect of temperature on removal of trace organic chemicals in managed aquifer recharge systems. Chemosphere 2015, 122, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Peldszus, S.; Elhadidy, A.M.; Legge, R.L.; Van Dyke, M.I.; Huck, P.M. Kinetics of natural organic matter (NOM) removal during drinking water biofiltration using different NOM characterization approaches. Water Res. 2016, 104, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vasyukova, E.; Proft, R.; Uhl, W. Evaluation of dissolved organic matter fractions removal due to biodegradation. In Progress in Slow Sand and Alternative Biofiltration Processes: Further Developments and Applications; IWA Publishing: London, UK, 2014; pp. 59–66. [Google Scholar]
- So, S.H.; Choi, I.H.; Kim, H.C.; Maeng, S.K. Seasonally related effects on natural organic matter characteristics from source to tap in Korea. Sci. Total Environ. 2017, 592, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Yang, L.; Guo, W.; Hong, H.; Wang, G. Non-conservative behaviors of chromophoric dissolved organic matter in a turbid estuary: Roles of multiple biogeochemical processes. Estuar. Coast. Shelf Sci. 2013, 133, 285–292. [Google Scholar] [CrossRef]
- Yang, L.; Shin, H.-S.; Hur, J. Estimating the Concentration and Biodegradability of Organic Matter in 22 Wastewater Treatment Plants Using Fluorescence Excitation Emission Matrices and Parallel Factor Analysis. Sensors 2014, 14, 1771–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Y.; Koal, P.; Gerl, G.; Schroll, R.; Joergensen, R.G.; Munch, J.C. Water-extractable organic matter and its fluorescence fractions in response to minimum tillage and organic farming in a Cambisol. Chem. Biol. Technol. Agric. 2017, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Gimbel, R.; Graham, N.J.D.; Collins, M.R. Recent Progress in Slow sand and Alternative Biofiltration Processes; IWA Publishing: London, UK, 2006. [Google Scholar]
- Wang, Y. Assessment of Ozonation and Biofiltration as a Membrane Pre-Treatment at a Full-Scale Drinking Water Treatment Plant; UWSpace: Waterloo, ON, Canada, 2014. [Google Scholar]
- Gerlach, M.; Gimbel, R. Influence of humic substance alteration during soil passage on their treatment behaviour. Water Sci. Technol. 1999, 40, 231–239. [Google Scholar] [CrossRef]







| Unit | DC | DCWW | WW | WEOM | |
|---|---|---|---|---|---|
| pH | - | 7.87 | 7.79 | 7.66 | 7.65 |
| DOC | mg·L−1 | 11.6 ± 0.7 | 10.5 ± 0.4 | 9.7 ± 0.6 | 14.6 ± 1.6 |
| SUVA254 | L·mg−1·m−1 | 2.84 ± 0.33 | 2.37 ± 0.28 | 2.56 ± 0.42 | 3.56 ± 0.71 |
| NO3-N | mg-N·L−1 | 2.06 ± 0.27 | 2.10 ± 0.19 | 1.87 ± 0.15 | 4.03 ± 0.43 |
| NH4-N | mg-N·L−1 | 0.24 ± 0.04 | 0.21 ± 0.07 | 0.17 ± 0.03 | 0.31 ± 0.06 |
| Mn | µg·L−1 | 46.8 | 14 | 14.03 | 86.74 |
| Fe | µg·L−1 | 175 | 87.4 | 37.6 | 109.6 |
| Zn | µg·L−1 | 20.9 | 30.1 | 36.6 | 36.6 |
| 30 °C | 25 °C | 20 °C | ||||
|---|---|---|---|---|---|---|
| Biotic | Abiotic | Biotic | Abiotic | Biotic | Abiotic | |
| DC | 10.5 ± 0.21 | 11.4 ± 0.35 | 9.76 ± 0.0.28 | 10.89 ± 0.61 | 9.24 ± 0.32 | 10.4 ± 0.43 |
| DCWW | 9.3 ± 0.18 | 10.29 ± 0.33 | 9.24 ± 0.37 | 10.1 ± 0.23 | 8.18 ± 0.17 | 9.4 ± 0.29 |
| WW | 8.27 ± 0.24 | 9.42 ± 0.51 | 7.95 ± 0.27 | 9.37 ± 0.23 | 7.37 ± 0.19 | 8.96 ± 0.18 |
| WEOM | 8.18 ± 0.26 | 12.87 ± 0.37 | 7.74 ± 0.41 | 11.7 ± 0.53 | 6.8 ± 0.27 | 10.1 ± 0.41 |
| DC | WEOM | |||||
|---|---|---|---|---|---|---|
| pH | DOC | SUVA254 | pH | DOC | SUVA | |
| - | (mg·L−1) | (L·mg−1·m−1) | - | (mg·L−1) | (L·mg−1·m−1) | |
| Influent | 7.82 | 10.80 ± 0.51 | 3.05 ± 0.31 | 7.73 | 14.16 ± 0.73 | 3.67 ± 0.21 |
| effluent-oxic | 7.91 | 9.49 ± 0.36 | 4.01 ± 0.24 | 7.88 | 7.91 ± 0.17 | 3.92 ± 0.14 |
| effluent-anoxic | 8.08 | 10.12 ± 0.25 | 3.06 ± 0.19 | 8.16 | 9.37 ± 0.28 | 3.73 ± 0.33 |
| effluent-anaerobic | 8.13 | 10.26 ± 0.55 | 3.21 ± 0.27 | 7.95 | 10.08 ± 0.39 | 3.67 ± 0.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrady, A.; Sharma, S.; Sefelnasr, A.; Kennedy, M. The Fate of Dissolved Organic Matter (DOM) During Bank Filtration under Different Environmental Conditions: Batch and Column Studies. Water 2018, 10, 1730. https://doi.org/10.3390/w10121730
Abdelrady A, Sharma S, Sefelnasr A, Kennedy M. The Fate of Dissolved Organic Matter (DOM) During Bank Filtration under Different Environmental Conditions: Batch and Column Studies. Water. 2018; 10(12):1730. https://doi.org/10.3390/w10121730
Chicago/Turabian StyleAbdelrady, Ahmed, Saroj Sharma, Ahmed Sefelnasr, and Maria Kennedy. 2018. "The Fate of Dissolved Organic Matter (DOM) During Bank Filtration under Different Environmental Conditions: Batch and Column Studies" Water 10, no. 12: 1730. https://doi.org/10.3390/w10121730
APA StyleAbdelrady, A., Sharma, S., Sefelnasr, A., & Kennedy, M. (2018). The Fate of Dissolved Organic Matter (DOM) During Bank Filtration under Different Environmental Conditions: Batch and Column Studies. Water, 10(12), 1730. https://doi.org/10.3390/w10121730