Towards a Sustainable Water Supply: Humic Acid Removal Employing Coagulation and Tangential Cross Flow Microfiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Powdered Chemicals, Chemical Solutions, and Polymer Coagulants
2.2. Methods
2.2.1. Experimental
Microfiltration Membrane
Microfiltration Unit Design
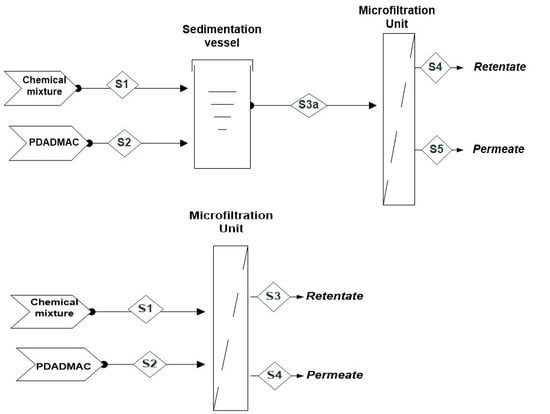
2.2.2. Processing Schemes
Sequential Coagulation–Microfiltration
Hybrid Coagulation–Microfiltration
2.2.3. Analysis of Physicochemical Characteristics
2.3. Theoretical
Determination of the Filtration Parameters
3. Results and Discussion
3.1. Retention of Humic Acid under Various Salts Concentrations
3.2. Retention of Humic Acid under Various Metals Concentrations
3.3. Effect of Processing Schemes in the Membrane Flux
3.4. Effect of Processing Schemes in the Membrane Structure
4. Conclusions
- Ceramic tangential cross flow microfiltration combined with coagulation is an effective treatment for humic acid removal.
- Two processing schemes were tested, namely sequential coagulation and microfiltration and hybrid coagulation and microfiltration. Hybrid coagulation and microfiltration is a highly effective treatment scheme in all cases, with an elevated flux when compared with sequential coagulation and microfiltration.
- Several hydrodynamic conditions namely transmembrane pressure and flowrate were tested, concluding that 1.5 bar of transmembrane pressure offers the higher humic acid retention.
- Hybrid coagulation and microfiltration has also the least effect of the membrane surface compared with the other processing scheme, promoting longevity of the membrane equipment used.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.prb.org/Populationtrendsandchallengesinthemiddleeastandnorthafrica/ (accessed on 29 August 2019).
- Alkhudhiri, A.; Darwish, N.B.; Hilal, N. Analytical and Forecasting Study for Wastewater Treatment and Water Resources in Saudi Arabia. J. Water Process Eng. 2019, 32. [Google Scholar] [CrossRef]
- El-Ghonemy, A.M.K. A small-scale brackish water reverse-osmosis desalination system used in northern Saudi Arabia: A case study. Renew. Sustain. Energy Rev. 2012, 16, 4597–4605. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviors of natural organic matter in membrane filtration for surface water treatment—A Review. Desal 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Humic acid fouling during microfiltration. J. Membr. Sci. 1999, 157, 1–12. [Google Scholar] [CrossRef]
- Zeng, K.; Hwang, H.-M.; Yuzuri, H. Effect of dissolved humic substances on the photochemical degradation rate of 1-aminopyrene and atrazine. Int. J. Mol. Sci. 2000, 3, 1048–1057. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2004, 22, 45–56. [Google Scholar] [CrossRef]
- Edzwald, J.K. American Water Works Association (AWWA) Water Quality & Treatment: A Handbook on Drinking Water, 6th ed.; McGraw-Hill Education-Europe, McGraw-Hill Professional: Denver, CO, USA, 2000. [Google Scholar]
- Capodaglio, A.G. Integrated, Decentralized Wastewater Management for Resource Recovery in Rural and Peri-Urban Areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Tech. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. The filtration characteristics of anaerobic digester effluents employing cross flow ceramic membrane microfiltration for nutrient recovery. Desal 2014, 341, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Zacharof, M.P.; Mandale, S.J.; Oatley-Radcliffe, D.; Lovitt, R.W. Nutrient recovery and fractionation of anaerobic digester effluents employing pilot scale membrane technology. J. Water Process Eng. 2019, 31. [Google Scholar] [CrossRef]
- Cabero, M.; Riera, F.; Alvarez, R. Rinsing of ultrafiltration ceramic membranes fouled with whey proteins: Effects on cleaning procedures. J. Membr. Sci. 1999, 154, 239–250. [Google Scholar] [CrossRef]
- Mulder, M. The Use of Membrane Processes in Environmental Problems. An Introduction; Crespo, J.G., Böddeker, K.W., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 229–262. Nato Asi Series E. [Google Scholar]
- Costa, A.R.; De Pinho, M.N. The role of membrane morphology on ultrafiltration for natural organic matter removal. Desal 2002, 145, 299–304. [Google Scholar] [CrossRef]
- Xu, W.; Chellam, S.; Clifford, D.A. Indirect evidence for deposit rearrangement during dead-end microfiltration of iron coagulated suspensions. J. Membr. Sci. 2004, 239, 243–254. [Google Scholar] [CrossRef]
- Hakami, M.W.; Tizaoui, C.; Kochkodan, V.N.; Hilal, N. Effect of hydrodynamic operations, salinity, and heavy metals on ha removal by microfiltration ceramic tubular membrane. Sep. Sci. Tech. 2013, 48, 564–570. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Abri, M.; Al-Hinai, H.; Abu-Arabi, M. Characterization and retention of NF membranes using PEG, HS and polyelectrolytes. Desal 2008, 221, 284–293. [Google Scholar] [CrossRef]
- Kimura, K.; Iwase, T.; Kita, S.; Watanabe, Y. Influence of residual organic macromolecules produced in biological wastewater treatment processes on removal of pharmaceutical by NF/RO membranes. Water Res. 2009, 43, 3751–3758. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Alvarez, S.; Riera, F.A.; Alvarez, R. Lactic acid recovery from whey ultrafiltrate fermentation broths and artificial solutions by nanofiltration. Desal 2008, 228, 84–96. [Google Scholar] [CrossRef]
- Wagner, J. Membrane Filtration Handbook—Practical Tips and Hints, 2nd ed.; Osmonics: Minnetonka, MN, USA, 2001; pp. 1–127. [Google Scholar]
- Al-Abri, M.; Hilal, N. Artificial neural network simulation of combined humic substance coagulation and membrane filtration. Chem. Eng. J. 2008, 141, 27–34. [Google Scholar] [CrossRef]
- Al-Abri, M.; Dakheel, A.; Tizaoui, C.; Hilal, N. Combined humic substance and heavy metals coagulation, and membrane filtration under saline conditions. Desal 2010, 253, 46–50. [Google Scholar] [CrossRef]
- Lowe, J.; Hossain, M.M. Application of ultrafiltration membranes for removal of humic acid from drinking water. Desal 2008, 218, 343–354. [Google Scholar] [CrossRef]
- Sutzkover-Gutman, I.; Hasson, D.; Semiat, R. Humic substances fouling in ultrafiltration processes. Desal 2010, 261, 218–231. [Google Scholar] [CrossRef]
- Costa, A.R.; De Pinho, M.N. Effect of membrane pore size and solution chemistry on the ultrafiltration of humic substances solutions. J. Membr. Sci. 2005, 255, 49–56. [Google Scholar] [CrossRef]
- Jones, K.L.; O’Melia, C.R. Protein and humic acid adsorption onto hydrophilic membrane surfaces: Effects of pH and ionic strength. J. Membr. Sci. 2000, 165, 31–46. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Mauch, R.; Waite, T.D.; Fane, A.G. Charge effects in the fractionation of natural organics using ultrafiltration. Environ. Sci. Tech. 2002, 36, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Thomassen, J.K.; Faraday, D.B.F.; Underwood, B.O.; Cleaver, J.A.S. The effect of varying transmembrane pressure and crossflow velocity on the microfiltration fouling of a model beer. Sep. Purif. Tech. 2005, 41, 91–100. [Google Scholar] [CrossRef]
- Zularisam, A.; Ismail, A.; Sakinah, M. Application and challenges of membrane in surface water treatment. J. Appl. Sci. 2010, 10, 380–390. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Valenzuela-Calahorro, C.; Garrido, J.J. Cu(II) retention on a humic substance. J. Colliod Interface Sci. 2004, 270, 47–55. [Google Scholar] [CrossRef]
- Zhou, P.; Yan, H.; Gu, B. Competitive complexation of metal ions with humic substances. Chemosphere 2005, 58, 1327–1337. [Google Scholar] [CrossRef]
- Hankins, N.P.; Lu, N.; Hilal, N. Enhanced removal of heavy metal ions bound to humic acid by polyelectrolyte flocculation. Sep. Purif. Tech. 2006, 51, 48–56. [Google Scholar] [CrossRef]
- Brigante, M.; Zanini, G.; Avena, M. Effect of pH, anions and cations on the dissolution kinetics of humic acid particles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 180–186. [Google Scholar] [CrossRef]
- Kloster, N.; Brigante, M.; Zanini, G.; Avena, M. Aggregation kinetics of humic acids in the presence of calcium ions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 427, 76–82. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Shaker, M.; El-Toukhy, A.; Abid, I.M.; Davies, G. Thermodynamics of metal cation binding by a solid soil-derived humic acid: Binding of Fe(III), Pb(II), And Cu(II). Chemosphere 2006, 63, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ghabbour, E.A.; Shaker, M.; El-Toukhy, A.; Abid, I.M.; Davies, G. Thermodynamics of metal cation binding by a solid soil derived humic acid. 2. Binding of Mn(II), and Hg(II). Chemosphere 2006, 64, 826–833. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Coustumer, P.L. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 48–55. [Google Scholar] [CrossRef]
- Konieczny, K.; Bodzek, M.; Rajca, M. A coagulation–MF system for water treatment using ceramic membranes. Desal 2006, 198, 92–101. [Google Scholar] [CrossRef]
- Nishi, L.; Vieira, A.M.S.; Vieira, M.F.; Silva, G.F.; Bergamasco, R. Application of hybrid process of coagulation/flocculation and membrane filtration for the removal of protozoan parasites from water. Proced. Eng. 2012, 42, 148–160. [Google Scholar] [CrossRef]
- Wershaw, R.L. Application of a membrane model to the sorptive interactions of humic substances. Environ. Health Perspect. 1989, 83, 191–203. [Google Scholar] [CrossRef]
- Konieczny, K.; Rajca, M.; Bodzek, M.; Kwiecińska, A. Water treatment using hybrid method of coagulation and low-pressure membrane filtration. Environ. Prot. Eng. 2009, 35, 5–22. [Google Scholar]
- Amy, G. Fundamental understanding of organic matter fouling of membranes. Desal 2008, 231, 44–51. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Abu Arabi, M. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desal 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Zahrim, A.Y.; Tizaoui, C.; Hilal, N. Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desal 2011, 266, 1–16. [Google Scholar] [CrossRef]
- Johnson, D.J.; Miles, N.J.; Hilal, N. Quantification of particle–bubble interactions using atomic force microscopy: A review. Adv. Colloid Interface Sci. 2006, 127, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Al Malek, S.; Al-Rashdi, B.; Hilal, N. Atomic force microscopy of nanofiltration membranes: Effect of imaging mode and environment. J. Membr. Sci. 2012, 389, 486–498. [Google Scholar] [CrossRef]







| Solution Composition: Humic Acid 10 mg/L, 1 mg/L PDADMAC (for Sequential and Hybrid Processes Only) pH 7, TMP 1.5 bar | Flux (J, m3/m2 s) | |||
| Microfiltration | Sequential Coagulation-Microfiltration | Hybrid Coagulation-Microfiltration | ||
| Salinity (NaCl, g/L) | ||||
| 10 | 1.91 × 10−5 | 0.05 | 0.13 | |
| 20 | 1.70 × 10−5 | 0.06 | 0.11 | |
| 35 | 1.30 × 10−5 | 0.08 | 0.1 | |
| Heavy Metals (mg/L) | ||||
| 5 | 5.48 × 10−5 | 0.13 | 0.15 | |
| 10 | 6.98 × 10−5 | 0.11 | 0.14 | |
| Membrane Condition (MF 0.5 μm) | Mean Roughness (Ra, nm) | Root Mean Square (RMS, nm) | Surface Area Difference (%) | Max. Range (nm) | Mean Max. Range (nm) |
|---|---|---|---|---|---|
| Untreated dry | 24.81 | 33.75 | 19.11 | 253.10 | 183.19 |
| Treated (NaOH 1% v/v) | 7.22 | 10.00 | 4.97 | 148.13 | 71.68 |
| Fouled (Sequential Coagulation-MF) | 4.10 | 5.55 | 17.05 | 86.99 | 59.34 |
| Fouled (Hybrid Coagulation-MF) | 7.82 | 9.82 | 5.53 | 76.245 | 65.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakami, M.W.; Alkhudhiri, A.; Zacharof, M.-P.; Hilal, N. Towards a Sustainable Water Supply: Humic Acid Removal Employing Coagulation and Tangential Cross Flow Microfiltration. Water 2019, 11, 2093. https://doi.org/10.3390/w11102093
Hakami MW, Alkhudhiri A, Zacharof M-P, Hilal N. Towards a Sustainable Water Supply: Humic Acid Removal Employing Coagulation and Tangential Cross Flow Microfiltration. Water. 2019; 11(10):2093. https://doi.org/10.3390/w11102093
Chicago/Turabian StyleHakami, Mohammed Wali, Abdullah Alkhudhiri, Myrto-Panagiota Zacharof, and Nidal Hilal. 2019. "Towards a Sustainable Water Supply: Humic Acid Removal Employing Coagulation and Tangential Cross Flow Microfiltration" Water 11, no. 10: 2093. https://doi.org/10.3390/w11102093
APA StyleHakami, M. W., Alkhudhiri, A., Zacharof, M. -P., & Hilal, N. (2019). Towards a Sustainable Water Supply: Humic Acid Removal Employing Coagulation and Tangential Cross Flow Microfiltration. Water, 11(10), 2093. https://doi.org/10.3390/w11102093