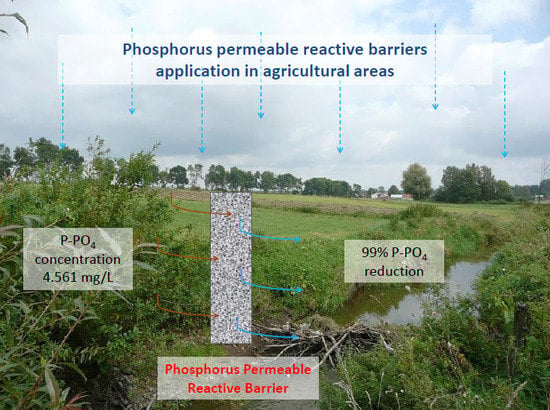
Permeable Reactive Barriers for Preventing Water Bodies from a Phosphorus-Polluted Agricultural Runoff-Column Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Reactive Materials
2.3. Leaching Experiment
2.4. Statistical Analysis
3. Results
3.1. Leakages Quantity
3.2. Leakages Quality
3.3. P Sorption
4. Discussion
4.1. Reactive Materials Sorption Limitations
4.2. Permeable Reactive Barrier Efficiency and Dimensioning
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Obolewski, K.; Glińska-Lewczuk, K.; Bąkowska, M.; Szymańska, M.; Mrozińska, N. Patterns of phytoplankton composition in coastal lakes differed by connectivity with the Baltic Sea. Sci. Total Environ. 2018, 631–632, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-H.; Watson, C.C.; Biedenharn, D.S.; Carlson, K.H. Reactive Stream Stabilization for Minimizing Transport of Phosphorus and Nitrogen from Agricultural Landscapes. J. Water Resour. Prot. 2011, 3, 504–512. [Google Scholar] [CrossRef]
- Haygarth, P.M.; Jarvis, S.C. Agriculture, Hydrology and Water Quality; Haygarth, P.M., Jarvis, S.C., Eds.; CABI: Wallingford, CT, USA, 2002; ISBN 978-0-85199-545-8. [Google Scholar]
- Hewelke, E.; Szaty, J.; Gnatowski, T.; Oleszczuk, R. Spatial Variability in Soil Moisture Content under Preferential Flow in Hydrophobic Organic Soil. Ann. Set Environ. Prot. 2014, 16, 580–607. [Google Scholar]
- Verheyen, D.; Van Gaelen, N.; Ronchi, B.; Batelaan, O.; Struyf, E.; Govers, G.; Merckx, R.; Diels, J. Dissolved phosphorus transport from soil to surface water in catchments with different land use. AMBIO 2015, 44, 228–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, A.; Madramootoo, C.A.; Enright, P. Phosphorus losses in surface and subsurface runoff from a snowmelt event on an agricultural field in Quebec. Can. Biosyst. Eng. 2003, 45, 7. [Google Scholar]
- Kleinman, P.; Sharpley, A.; Buda, A.; McDowell, R.; Allen, A. Soil controls of phosphorus in runoff: Management barriers and opportunities. Can. J. Soil Sci. 2011, 91, 329–338. [Google Scholar] [CrossRef]
- Ulén, B.; Larsbo, M.; Koestel, J.; Hellner, Q.; Blomberg, M.; Geranmayeh, P. Assessing strategies to mitigate phosphorus leaching from drained clay soils. AMBIO 2018, 47, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Szejba, D.; Papierowska, E.; Cymes, I.; Bankowska, A. Nitrate nitrogen and phosphate concentrations in drainflow: An example of clay soil. J. Elem. 2016, 21. [Google Scholar] [CrossRef]
- Pulikowski, K.; Pawęska, K.; Bawiec, A. Seasonal changes in phosphorus load flowing out of small agricultural catchments. J. Ecol. Eng. 2015, 16, 81–86. [Google Scholar] [CrossRef]
- Ulén, B.; Stenberg, M.; Wesström, I. Use of a flashiness index to predict phosphorus losses from subsurface drains on a Swedish farm with clay soils. J. Hydrol. 2016, 533, 581–590. [Google Scholar] [CrossRef]
- Grizzetti, B.; Bouraoui, F.; Aloe, A. Changes of nitrogen and phosphorus loads to European seas. Glob. Chang. Biol. 2012, 18, 769–782. [Google Scholar] [CrossRef]
- OSPAR. Data Report on the Comprehensive Study of Riverine Inputs and Direct Dis-Charges (RID) in 2000; OSPAR Commission: London, UK, 2002. [Google Scholar]
- Helsinki Commission. Fourth Baltic Sea Load Compilation; Baltic Sea Environment Protection No. 93; Helsinki Commission: Washington, DC, USA, 2003.
- UNEP/MAP/WHO. Assessment of Eutrophication in the Mediterranean Sea MAP; Technical Reports Series No. 106; UNEP/MAP: Athens, Greece, 1996. [Google Scholar]
- ICPDR. Water Quality in the Danube River Basin; TNMN-Yearbook; ICPDR: Vienna, Austria, 2000. [Google Scholar]
- Bakan, G.; Buyukgungor, H. The Black Sea. Mar. Pollut. Bull. 2000, 41, 24–43. [Google Scholar] [CrossRef]
- USEPA. Permeable Reactive Barrier Technologies for Contaminant Remediation; EPA/600/R-98/125; United States Environmental Protection Agency: Washington, DC, USA, 1998.
- Jacobs, P.H.; Förstner, U. Concept of subaqueous capping of contaminated sediments with active barrier systems (ABS) using natural and modified zeolites. Water Res. 1999, 33, 2083–2087. [Google Scholar] [CrossRef]
- Ibrahim, T.G.; Goutelle, A.; Healy, M.G.; Brennan, R.; Tuohy, P.; Humphreys, J.; Lanigan, G.; Brechignac, J.; Fenton, O. Mixed Agricultural Pollutant Mitigation Using Woodchip/Pea Gravel and Woodchip/Zeolite Permeable Reactive Interceptors. Water Air Soil Pollut. 2015, 226, 51. [Google Scholar] [CrossRef]
- Canga, E.; Heckrath, G.J.; Kjaergaard, C. Agricultural Drainage Filters. II. Phosphorus Retention and Release at Different Flow Rates. Water Air Soil Pollut. 2016, 227, 276. [Google Scholar] [CrossRef]
- Miller, M.L.; Bhadha, J.H.; O’Connor, G.A.; Jawitz, J.W.; Mitchell, J. Aluminum water treatment residuals as permeable reactive barrier sorbents to reduce phosphorus losses. Chemosphere 2011, 83, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y.; Zhu, Z. Evaluation of sediment capping with active barrier systems (ABS) using calcite/zeolite mixtures to simultaneously manage phosphorus and ammonium release. Sci. Total Environ. 2011, 409, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, K.; Fronczyk, J. Evaluation of single and multilayered reactive zones for heavy metals removal from stormwater. Environ. Technol. 2015, 36, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.R.; Pascu, D.E.; Orbulet, O.D.; Modrogan, C.; Neagu (Pascu), M.; Popa, G.A. The use of permeable reactive barriers for removal of phosphate from ground waters. In Proceedings of the 3rd International Conference Research & Innovation in Engineering, Braşov, Romania, 16–17 October 2014. [Google Scholar]
- Adera, S.; Drizo, A.; Twohig, E.; Jagannathan, K.; Benoit, G. Improving Performance of Treatment Wetlands: Evaluation of Supplemental Aeration, Varying Flow Direction, and Phosphorus Removing Filters. Water Air Soil Pollut. 2018, 229, 100. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A.; Baryła, A. Calcined Eggshell as a P Reactive Media Filter—Batch Tests and Column Sorption Experiment. Water Air Soil Pollut. 2019, 230, 20. [Google Scholar] [CrossRef] [PubMed]
- Bus, A.; Karczmarczyk, A. Supporting constructed wetlands in P removal efficiency from surface water. Water Sci. Technol. 2017, 75, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Bus, A.; Baryła, A. Phosphate Leaching from Green Roof Substrates—Can Green Roofs Pollute Urban Water Bodies? Water 2018, 10, 199. [Google Scholar] [CrossRef]
- Karapınar, N. Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. J. Hazard. Mater. 2009, 170, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Kirkkala, T.; Ventelä, A.-M.; Tarvainen, M. Long-Term Field-Scale Experiment on Using Lime Filters in an Agricultural Catchment. J. Environ. Qual. 2012, 41, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Kirkkala, T.; Ventelä, A.-M.; Tarvainen, M. Fosfilt filters in an agricultural catchment: A long-term field-scale. Agric. Food Sci. 2012, 21, 237–246. [Google Scholar] [CrossRef]
- Izydorczyk, K.; Frątczak, W.; Drobniewska, A.; Cichowicz, E.; Michalska-Hejduk, D.; Gross, R.; Zalewski, M. A biogeochemical barrier to enhance a buffer zone for reducing diffuse phosphorus pollution—Preliminary results. Ecohydrol. Hydrobiol. 2013, 13, 104–112. [Google Scholar] [CrossRef]
- PN EN 1097-3:2000 Equivalent to EN 1097-3:1998 Tests for Mechanical and Physical Properties of Aggregates—Determination of Loose Bulk Density and Voids; British Standards Institution: London, UK, 1998.
- Zak, D.; Gelbrecht, J.; Wagner, C.; Steinberg, C.E.W. Evaluation of phosphorus mobilization potential in rewetted fens by an improved sequential chemical extraction procedure. Eur. J. Soil Sci. 2018, 59, 1191–1201. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Naidu, R. Permeable reactive barrier for groundwater remediation. J. Ind. Eng. Chem. 2008, 14, 145–156. [Google Scholar] [CrossRef]
- PN EN 933–1:2012 Equivalent to EN 933–1:2012 Tests for Geometrical Properties of Aggregates. Determination of Particle Size Distribution; Sieving Method; British Standards Institution: London, UK, 2012.
- International Organization for Standardization (ISO). PN-ISO 11277:2005 Equivalent to ISO 11277:2009 Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- PN-EN 1936:2010 Equivalent to EN 1936:2006 Natural Stone Test Methods. Determination of Real Density and Apparent Density, and of Total and Open Porosity; British Standards Institution: London, UK, 2007.
- PN-EN 1097-6:2013-11, Tests for Mechanical and Physical Properties of Aggregates. Determination of Grain Density and Water Absorption; Polish Standards Institution: Warsaw, Poland, 2013.
- Bus, A.; Karczmarczyk, A. Kinetic studies on removing phosphate from synthetic solution and river water by reactive material in a form of suspended reactive filters. Desalination Water Treat. 2018, 136, 237–244. [Google Scholar] [CrossRef]
- Cucarella, V.; Renman, G. Phosphorus Sorption Capacity of Filter Materials Used for On-site Wastewater Treatment Determined in Batch Experiments—A Comparative Study. J. Environ. Qual. 2009, 38, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Cucarella, V.; Zaleski, T.; Mazurek, R. Phosphorus sorption capacity of different types of opoka. Ann. Wars. Univ. Life Sci.-SGGW Land Reclam. 2007, 38, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Buriánek, P.; Skalický, M.; Grünwald, A. Phosphates Adsorption from Water by Recycled Concrete/Adsorpce Fosforečnanů Z Vody Recyklovaným Betonem. Geosci. Eng. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Renman, G.; Renman, A. Sustainable Use of Crushed Autoclaved Aerated Concrete (CAAC) as a Filter Medium in Wastewater Purification; ISCOWA and SGI: Gothenburg, Sweden, 2012. [Google Scholar]
- Johansson, L. Industrial By-Products and Natural Substrata as Phosphorus Sorbents. Environ. Technol. 1999, 20, 309–316. [Google Scholar] [CrossRef]
- Drizo, A.; Frost, C.A.; Grace, J.; Smith, K.A. Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems. Water Res. 1999, 33, 3595–3602. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.; He, Y.; Zhang, B.; Kirumba, G.; Xie, J. Adsorptive removal of phosphorus from aqueous solution using sponge iron and zeolite. J. Colloid Interface Sci. 2013, 402, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Blowes, D.W.; Ptacek, C.J.; Benner, S.G.; McRae, C.W.; Bennett, T.A.; Puls, R.W. Treatment of inorganic contaminants using permeable reactive barriers. J. Contam. Hydrol. 2000, 45, 123–137. [Google Scholar] [CrossRef]
- Ballantine, D.J.; Tanner, C.C. Substrate and filter materials to enhance phosphorus removal in constructed wetlands treating diffuse farm runoff: A review. N. Z. J. Agric. Res. 2010, 53, 71–95. [Google Scholar] [CrossRef]
- Bus, A.; Mosiej, J. Water Quality Changes of Inflowing and Outlawing Water from Complex of Niewiadoma Reservoirs Located at Cetynia River. Ann. Set Environ. Prot. 2019, 20, 1793–1810. [Google Scholar]
- McDowell, R.W.; Sharpley, A.N.; Bourke, W. Treatment of drainage water with industrial by-products to prevent phosphorus loss from tile-drained land. J. Environ. Qual. 2008, 37, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- EEA. The European Environment—State and Outlook 2010: Synhesis; European Environment Agency: Copenhagen, Denmark, 2010. [Google Scholar]
- Ulén, B.; Pietrzak, S.; Tonderski, K. Self-Assessment of Farms in the Field of Management of Fertilizer Components and Assessment of Environmental Conditions; ITP: Raszyn, Poland, 2013. [Google Scholar]
- HELCOM. Input of Nutrients by the Seven Biggest Rivers in the Baltic Sea Region; Baltic Sea Environment Proceedings No.161; HELCOM: Helsinki, Finland, 2018. [Google Scholar]




| Granulometric Composition | [%] |
|---|---|
| Sand | 77 |
| Silt | 13 |
| Colloidal clay | 7 |
| Organic matter [%] | 3 |
| Hydraulic conductivity [m/d] | 636 |
| Bulk density [g/cm3] | 1.46 |
| HCl extractable phosphorus [mg P/kg dry mass] | 236.56 |
| Zeolite | Polonite® | AAC | Limestone | |
|---|---|---|---|---|
| SiO2 | 47.80 | 55.11 | 57.24 | 0.61 |
| CaO | 2.84 | 23.86 | 24.62 | 29.30 |
| Al2O3 | 6.07 | 5.65 | 1.96 | |
| K2O | 2.19 | 1.04 | 0.48 | |
| P2O5 | 0.18 | 0.31 | ||
| Fe | 1.07 | 0.14 | ||
| Fe2O3 | 2.10 | 0.14 | ||
| MgO | 0.52 | 6.79 | ||
| SO3 | 1.35 | |||
| Ti | 0.14 | |||
| Cl | 0.10 |
| Zeolite | Polonite® | AAC | Limestone | |
|---|---|---|---|---|
| Grain size [mm] | 1-2 | 1-2 | 1-2 | 1-2 |
| Porosity [%] | 50.0 | 55.0 | 65.0 | 40.0 |
| Bulk density [g/cm3] | 0.90 | 0.75 | 0.40 | 1.50 |
| Water adsorption [%] | 9.00 | 5.30 | 70.00 | 4.00 |
| RM | PRB Dimensions (W × D × L) | Cin [mg/L] | Removal [%] | “Lifetime” Efficiency [year] | Calculation Method | Reference |
|---|---|---|---|---|---|---|
| Punta Gorda Al-WTR | 1 m width | P-PO4: 1.0 | - | 4789 | Calculated based on Smax according to Darcy flow | [22] |
| P-PO4: 10.0 | - | 120 | ||||
| Manatee Al-WTR | P-PO4: 1.0 | - | 1367 | |||
| P-PO4: 10.0 | - | 34 | ||||
| Limestone | 1.5 × 1.5 × 10 | P-PO4: 0.02–7.71 | 58 | - | Pilot scale experiment in Zarzącin, Poland | [33] |
| Fosfilt-s | 5 × 1.5 × 15 | P-PO4: 0.666 | 45 | - | Pilot scale experiment in River Pyhäjoki catchment, Finland | [32] |
| TP: 0.698 | 37 | - | ||||
| Lime and sand | 0.5 × 1.0 × 30 | Before renovation: | - | Pilot scale experiments in River Yläneenjoki catchment, Finland | [31] | |
| P-PO4: 2.642 | 62.6 | |||||
| TP : 3.055 | 60.0 | |||||
| After renovation: | - | |||||
| P-PO4: 4.005 | 61.1 | |||||
| TP : 3.302 | 61.3 | |||||
| Burnt lime and sand | 10 × 0.9 × 50 | P-PO4: 0.011 | 52.0 | - | ||
| TP: 0.090 | 67.0 | - | ||||
| Burnt lime and spent lime | 7.5 × 0.9 × 50 | P-PO4: 0.009 | 46.0 | - | ||
| TP: 0.076 | 82.0 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bus, A.; Karczmarczyk, A.; Baryła, A. Permeable Reactive Barriers for Preventing Water Bodies from a Phosphorus-Polluted Agricultural Runoff-Column Experiment. Water 2019, 11, 432. https://doi.org/10.3390/w11030432
Bus A, Karczmarczyk A, Baryła A. Permeable Reactive Barriers for Preventing Water Bodies from a Phosphorus-Polluted Agricultural Runoff-Column Experiment. Water. 2019; 11(3):432. https://doi.org/10.3390/w11030432
Chicago/Turabian StyleBus, Agnieszka, Agnieszka Karczmarczyk, and Anna Baryła. 2019. "Permeable Reactive Barriers for Preventing Water Bodies from a Phosphorus-Polluted Agricultural Runoff-Column Experiment" Water 11, no. 3: 432. https://doi.org/10.3390/w11030432
APA StyleBus, A., Karczmarczyk, A., & Baryła, A. (2019). Permeable Reactive Barriers for Preventing Water Bodies from a Phosphorus-Polluted Agricultural Runoff-Column Experiment. Water, 11(3), 432. https://doi.org/10.3390/w11030432