Magnetic Field Usage Supported Filtration Through Different Filter Materials
Abstract
:1. Introduction
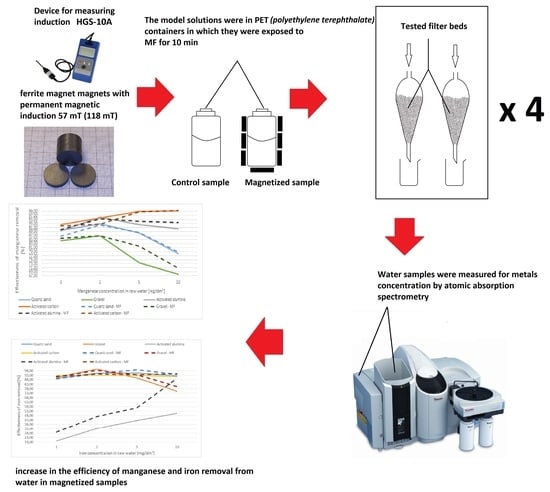
2. Materials and Methods
3. Results and Discussion
- worked out natural quartz masses, covered with a stable coating of iron and manganese oxides, formed from iron and manganese compounds removed from water during the filtration process,
- natural manganese ores—depending on the origin from 60 to 95% MnO2,
- deposits that are pre-activated at the production, covered with iron and manganese oxides according to patented technologies, e.g., with grains industrially coated with manganese dioxide (MnO2) coatings.
4. Conclusions
- Among the tested filtration materials, i.e., quartz sand, gravel, activated alumina and active carbon, the material most susceptible to MF influence turned out to be activated alumina, which allowed us to increase the removal efficiency of tested pollutants by about 1–3% compared to processes without the application of an MF
- From among all analyzed filtration masses used to remove iron from the raw model water, the best effect of Fe removal was obtained for filtration on quartz sand and gravel. The effect of the MF was best observed in the removal of iron in the filtration process on activated alumina.
- The most effective filter beds in manganese removal from the model raw water were activated carbon and aluminum oxide. In the case of manganese removal during the filtration process on quartz sand and gravel, a decrease in efficiency was observed along with an increase in the concentration of manganese in raw water from 0.2 mg/dm3 to 0.5 mg/dm3 and up to 1 mg/dm3.
- The magnetic field can support the filtration process, especially since it does not increase the number of chemical compounds introduced into water treatment systems or the environment. Experiments on the impact of MF on water purification processes should be continued in order to thoroughly investigate the impact.
Author Contributions
Funding
Conflicts of Interest
References
- Skoczko, I.; Szatyłowicz, E. Removal of heavy metal ions by filtration on activated alumina-assisted magnetic field. Desalin. Water Treat. 2018, 117, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Skórowski, Ł.; Olejniczak, G. The use of the Lemna gibba test in the assessment of magnetically conditioned water. Water Tech. 2017, 4, 22–27. (In Polish) [Google Scholar]
- Skórowski, Ł. Phytotoxicity assessment of water subjected to magnetic field. Ecol. Eng. 2017, 18, 142–148. (In Polish) [Google Scholar] [CrossRef]
- Fathi, A.; Mohamed, T.; Claude, G.; Maurin, G.; Mohanmed, B.A. Effect of a magnetic water treatment on homogeneous and heterogeneous precipitation of calcium carbonate. Water Res. 2006, 40, 1941. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Lee, S.H. Reduction in the surface tension of water due to physical water treatment for fouling control in heat exchangers. Int. Commun. Heat Mass. 2005, 32, 1–9. [Google Scholar] [CrossRef]
- Amiri, M.C.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Ghauri, S.A.; Ansari, M.S. Increase of water viscosity under the influence of magnetic field. J. Appl. Phys. 2006, 6, 100. [Google Scholar] [CrossRef]
- Toledo, E.J.L.; Ramalho, T.C.; Magriotis, Z.M. Influence of magnetic field on physical–chemical properties of the liquid water: Insights from experimental and theoretical models. J. Mol. Struct. 2008, 888, 409–415. [Google Scholar] [CrossRef]
- Deng, B.; Pang, X.F. Variations of optic properties of water under action of static magnetic field. Chin. Sci. Bull. 2007, 52, 3179–3182. [Google Scholar] [CrossRef]
- Szcześ, A.; Chibowski, E.; Hołysz, L.; Rafalski, P. Effects of static magnetic field on water at kinetic condition. Chem. Eng. Process. 2011, 50, 124–127. [Google Scholar] [CrossRef]
- Camper, A.K.; Brastrup, K.; Sandvig, A.; Clement, J.; Spencer, C.; Capuzzi, A.J. Effect of distribution system materials on bacterial regrowth. J. Am. Water Works Assoc. 2003, 7, 107–121. [Google Scholar] [CrossRef]
- Chibowski, E.; Hołysz, L.; Szcześ, A. Adhesion of in situ precipitated calcium carbonate in the presence and absence of magnetic field in quiescent conditions on different solid surfaces. Water Res. 2003, 37, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Alimia, F.; Tlili, M.M.; Ben Amora, M.; Maurin, G.; Gabrielli, C. Effect of magnetic water treatment on calcium carbonate precipitation: Influence of the pipe material. Chem. Eng. Process. 2009, 48, 1327–1332. [Google Scholar] [CrossRef]
- Bikul’chyus, G.; Ruchinskene, A.; Deninis, V. Corrosion Behavior of Low-Carbon Steel in Tap Water Treated with Permanent Magnetic Field. Prot. Met. 2003, 39, 443–447. [Google Scholar] [CrossRef]
- Brower, J. Magnetic Water Treatment. Pollut. Eng. 2005, 2, 26–28. [Google Scholar]
- Ricco, G.; Tomei, C.M.; Ramadori, R.; Laera, G. Toxicity assessment of common xenobiotic compounds on municipal activated sludge: Comparison between respirometry and Microtox. Water Res. 2004, 38, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.I.; Lane, J.; Kim, W. Pulsed-power treatment for physical water treatment. Int. Commun. Heat Mass. 2005, 32, 861–871. [Google Scholar] [CrossRef]
- Colic, M.; Morse, D.L. The elusive mechanism of the magnetic ‘memory’ of water. Colloid. Surf. A 1999, 154, 167–174. [Google Scholar] [CrossRef]
- Podsiadło, C.; Leśniak, E. Impact of magnetically treated water on germination and initial growth of selected plant species. Infrastruct. Ecol. Rural Areas 2009, 3, 213–221. (In Polish) [Google Scholar]
- Stankiewicz, T.; Kozak, K.; Podleśny, J.; Pietruszewski, S. Effect of magnetically treated water on germination and growth of narrow-leafed lupine seedlings. Acta Scientiarum Polonorum. Technica Agraria 2012, 11, 21–32. (In Polish) [Google Scholar]
- Łebkowska, M.; Rutkowska-Narożniak, A.; Pajor, E.; Pochanke, Z. Effect of a static magnetic field on formaldehyde biodegradation in wastewater by activated sludge. Bioresour. Technol. 2011, 102, 8777–8782. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Filipkowska, U.; Janczukowicz, W.; Kłodowska, I.; Prażmo, M. The influence of a permanent magnetic field on the transformation of nitrogen compounds in a biological disk deposit. Annu. Set Environ. Prot. 2013, 15, 1511–1524. (In Polish) [Google Scholar]
- Kenz, S.; Pohar, C. The magnetic field influence on the polymorph composition of CaCO3 precipitated from carbonized aqueous solutions. J. Colloid. Interf. Sci. 2005, 281, 377–388. [Google Scholar] [CrossRef]
- Thermo Scientific’s AAS iCE 3500 User Manual; Spectro-Lab: Warsaw, Poland, 2013.
- Skoczko, I.; Piekutin, J.; Roszczenko, A. Removal of iron and manganese compounds from water. Annu. Set Environ. Prot. 2015, 17, 1587–1608. (In Polish) [Google Scholar]
- Skoczko, I.; Szatyłowicz, E. Studies on the efficiency of groundwater treatment process with adsorption on activated alumina. Ecol. Eng. 2017, 18, 211–218. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpää, M. Magnetic field application and its potential in water and wastewater treatment systems. Sep. Purif. Technol. 2014, 43, 206–240. [Google Scholar] [CrossRef]
- Skoczko, I. Efficiency estimation of water purification with various filtration materials. Des. Water Treat. 2018, 134, 99–108. [Google Scholar] [CrossRef]
- Baker, J.S.; Judd, S.J. Magnetic amelioration of scale formation. Water Res. 1996, 30, 247–260. [Google Scholar] [CrossRef]
- Mahmoud, B.; Yosra, M.; Nadia, A. Effects of magnetic treatment on scaling power of hard waters. Sep. Purif. Technol. 2016, 171, 88–92. [Google Scholar] [CrossRef]
- Rusanowska, P.; Zieliński, M.; Dębowski, M. The influence of a constant magnetic field on the efficiency of fine bubble oxygenation of liquids. Ecol. Eng. 2017, 18, 130–135. [Google Scholar] [CrossRef]
- Jeż-Walkowiak, J.; Dymaczewski, Z.; Szuster-Janiaczyk, A.; Nowicka, A.B.; Szybowicz, M. Efficiency of Mn Removal of Different Filtration Materials for Groundwater Treatment Linking Chemical and Physical Properties. Water 2017, 9, 498. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, Q.; Qin, H.; Qiao, J.; Guan, X. The enhancing effect of weak magnetic field on degradation of Orange II by zero-valent iron. Desalin. Water Treat. 2016, 57, 1659–1670. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Chu, W.; Li, X.; Wei, H.; Gao, N. Weak magnetic field accelerates chloroacetamide removal by zero-valent iron in drinking water. Chem. Eng. J. 2019, 358, 40–47. [Google Scholar] [CrossRef]
- Tireli, A.A.; Firmino, M.F.C.; Oliveira, L.F.; Guimarães, I.R.; Guerreiro, M.C.; Silva, J.P. Influence of magnetic field on the adsorption of organic compound by clays modified with iron. Appl. Clay Sci. 2014, 97, 1–7. [Google Scholar] [CrossRef]
- González Vázquez, O.F.; Moreno Virgen, M.D.R.; Hernandez Montoya, V.; Tovar Gomez, R.; Alcantara Flores, J.L.; Pérez Cruz, M.A.; Montes Morán, M.A. Adsorption of Heavy Metals in the Presence of a Magnetic Field on Adsorbents with Different Magnetic Properties. Ind. Eng. Chem. Res. 2016, 55, 9323–9331. [Google Scholar] [CrossRef]
- Silva, I.B.; Queiroz, J.C.; Denise, N.; Petri, F.S. The effect of magnetic field on ion hydration and sulfate scale formation. Colloid Surf. A 2015, 465, 175–183. [Google Scholar] [CrossRef]
- Nakagawa, J.; Hirota, N.; Kitazawa, K.; Shoda, M. Magnetic field enhancement of water vaporization. J. Appl. Phys. 1999, 85, 2923–2925. [Google Scholar] [CrossRef]



| IDL (mg/dm3) | IQL (mg/dm3) | |
|---|---|---|
| Fe | 0.0043 | 0.050 |
| Mn | 0.0016 | 0.020 |
| TMDA 54,5 | AAS | Dev * | |||
|---|---|---|---|---|---|
| Concentration (mg/dm3) | ±SD (mg/dm3) | Concentration (mg/dm3) | ±SD (mg/dm3) | (%) | |
| Fe | 0.383 | 0.0325 | 0.377 | 0.042 | −1.57 |
| Mn | 0.287 | 0.0219 | 0.276 | 0.019 | −3.83 |
| Parameter | Raw Water | Quartz Sand | Gravel | Activated Alumina | Activated Carbon | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | CS | MF | CS | MF | CS | MF | CS | MF | |
| 1 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 0.95 | 0.099 ± 0.001 | 0.104 ± 0.001 | 0.091 ± 0.001 | 0.093 ± 0.002 | 0.767 ± 0.066 | 0.672 ± 0.022 | 0.067 ± 0.009 | 0.079 ± 0.004 |
| Redox (mV) | 305.1 | 237.8 | 183 | 224.5 | 243 | 111.5 | 102 | 88 | 16 |
| pH | 7.81 | 8.28 | 8.75 | 8.07 | 8.09 | 9.4 | 9.55 | 10.06 | 9.45 |
| Conductivity (µS/cm) | 18 | 52.1 | 54.9 | 32.9 | 44.8 | 239 | 205 | 1298 | 1080 |
| 2 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 1.92 | 0.089 ± 0.017 | 0.098 ± 0.036 | 0.002 ± 0.0009 | 0.031 ± 0.003 | 1.278 ± 0.18 | 1.0193 ± 0.26 | 0.138 ± 0.05 | 0.111 ± 0.07 |
| Redox (mV) | −23.1 | −26.2 | −5.2 | 132.4 | 5.6 | −36.1 | 27 | −21.1 | −5 |
| pH | 7.38 | 8.41 | 8.91 | 6.31 | 7.16 | 9.7 | 10.25 | 10.75 | 10.22 |
| Conductivity (µS/cm) | 53.1 | 61.4 | 61.4 | 81.5 | 86.2 | 364 | 296 | 1550 | 1293 |
| 5 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 4.89 | 0.216 ± 0.08 | 0.037 ± 0.07 | 0.487 ± 0.065 | 0.313 ± 0.026 | 2.813 ± 0.028 | 2.128 ± 0.105 | 0.3385 ± 0.005 | 0.312 ± 0.006 |
| Redox (mV) | 265.1 | 282 | 268.6 | 369 | 334.7 | 45.8 | 31.2 | −18.7 | −12 |
| pH | 5.56 | 5.44 | 6.19 | 4.38 | 4.72 | 9.78 | 9.8 | 10.51 | 9.81 |
| Conductivity (µS/cm) | 105.3 | 175.6 | 167.3 | 272 | 237 | 3222 | 341 | 1261 | 988 |
| 10 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 9.45 | 0.726 ± 0.051 | 0.559 ± 0.072 | 2.337 ± 0.097 | 1.843 ± 0.084 | 4.676 ± 0.092 | 1.001 ± 0.19 | 0.810 ± 0.035 | 0.494 ± 0.031 |
| Redox (mV) | 167.9 | 200.7 | 388 | 558.6 | 463.3 | 63.9 | 59.9 | 10.4 | 37.7 |
| pH | 3.80 | 3.77 | 4.46 | 3.39 | 3.55 | 9.32 | 9.59 | 10.04 | 8.68 |
| Conductivity (µS/cm) | 563 | 647 | 452 | 844 | 742 | 409 | 368 | 1041 | 752 |
| Parameter | Raw water | Quartz Sand | Gravel | Activated Alumina | Activated Carbon | ||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Sample | CS | MF | CS | MF | CS | MF | CS | MF | |
| 0.1 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 0.0841 | 0.0087 ± 0.001 | 0.0111 ± 0.003 | 0.013 ± 0.003 | 0.012 ± 0.003 | 0.009 ± 0.002 | 0.0084 ± 0.003 | 0.0063 ± 0.001 | 0.0069 ± 0.001 |
| Redox (mV) | 309.9 | 215 | 160.7 | 269.7 | 234.7 | 86.6 | 83.4 | 57.4 | 117 |
| pH | 6.71 | 8.04 | 9.03 | 7.11 | 7.49 | 9.42 | 9.33 | 10.08 | 8.66 |
| Conductivity (µS/cm) | 14 | 5.4 | 7.3 | 8.1 | 7.1 | 211 | 221 | 735 | 405 |
| 0.2 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 0.174 | 0.0124 ± 0.003 | 0.0135 ± 0.004 | 0.023 ± 0.003 | 0.023 ± 0.004 | 0.0066 ± 0.002 | 0.00819 ± 0.002 | 0.0072 ± 0.003 | 0.0127 ± 0.002 |
| Redox (mV) | 260.2 | 202.8 | 161.9 | 255.3 | 209.8 | 62.1 | 56.1 | 82.1 | 50.9 |
| pH | 5.35 | 8.25 | 8.64 | 6.9 | 7.9 | 9.69 | 9.88 | 10.15 | 9.45 |
| Conductivity (µS/cm) | 26 | 9.8 | 11.6 | 11.8 | 10.7 | 206 | 201 | 845 | 359 |
| 0.5 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 0.4636 | 0.0544 ± 0.021 | 0.052 ± 0.025 | 0.123 ± 0.046 | 0.085 ± 0.01 | 0.034 ± 0.003 | 0.0273 ± 0.004 | 0.004 ± 0.001 | 0.0049 ± 0.001 |
| Redox (mV) | 220.1 | 317.9 | 240.2 | 364.7 | 326.6 | 50.1 | 47.5 | 90.2 | 87.8 |
| pH | 6.9 | 6.81 | 7.36 | 5.17 | 5.92 | 9.38 | 9.29 | 9.98 | 9.35 |
| Conductivity (µS/cm) | 63.6 | 22.2 | 21.7 | 29.6 | 19.4 | 168.4 | 178.5 | 503 | 294 |
| 1 mg/dm3 | |||||||||
| Conc. (mg/dm3) | 0.8934 | 0.1958 ± 0.037 | 0.1889 ± 0.031 | 0.289 ± 0.046 | 0.2613 ± 0.034 | 0.0859 ± 0.043 | 0.0581 ± 0.015 | 0.0061 ± 0.001 | 0.0048 ± 0.001 |
| Redox (mV) | 229.2 | 257.9 | 208.9 | 389.3 | 368.4 | 62.7 | 45.6 | 64.4 | 107.5 |
| pH | 6.52 | 8.05 | 8.68 | 5.33 | 6.44 | 9.84 | 10.1 | 9.92 | 9.11 |
| Conductivity (µS/cm) | 132.1 | 44.3 | 45.5 | 67 | 51.3 | 172.3 | 182 | 480 | 263 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatyłowicz, E.; Skoczko, I. Magnetic Field Usage Supported Filtration Through Different Filter Materials. Water 2019, 11, 1584. https://doi.org/10.3390/w11081584
Szatyłowicz E, Skoczko I. Magnetic Field Usage Supported Filtration Through Different Filter Materials. Water. 2019; 11(8):1584. https://doi.org/10.3390/w11081584
Chicago/Turabian StyleSzatyłowicz, Ewa, and Iwona Skoczko. 2019. "Magnetic Field Usage Supported Filtration Through Different Filter Materials" Water 11, no. 8: 1584. https://doi.org/10.3390/w11081584
APA StyleSzatyłowicz, E., & Skoczko, I. (2019). Magnetic Field Usage Supported Filtration Through Different Filter Materials. Water, 11(8), 1584. https://doi.org/10.3390/w11081584