Impact of Slope of Growing Trays on Productivity of Wheat Green Fodder by a Nutrient Film Technique System
Abstract
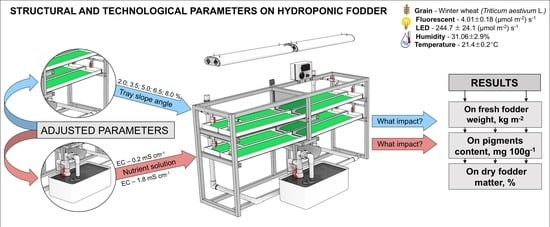
:1. Introduction
2. Materials and Methods
2.1. Upper Floor Lighting for Growing Trays
2.2. Lower Floor Lighting for Growing Trays
2.3. Preparation of Seeds before Germination and Cultivation of Wheat Fodder
2.4. Seed Spreading on Growing Trays and Watering
2.5. Nutrient Solution
2.6. Criteria for the Evaluation of Hydroponic Fodder
2.7. Fodder Yield
2.8. Determination of Dry Matter
2.9. Determination of Chlorophyll a and b and Carotenoids
2.10. Electricity Costs and Water Consumption
2.11. Economic Evaluation
2.12. Statistical Analysis
3. Results
3.1. Slope of Growing Trays
3.2. Nutrient Solution
3.3. Economic Evaluation
4. Discussion
Areas of Further Research
- At the beginning of the germination of wheat seeds, the slope angle (α) of the growing trays should (could) be lower (i.e., 2%). At that time, the seeds have no roots and no solid bedding. At the beginning of germination, if the trays are set at a higher angle (i.e., 6.5% or 8.0%) and watering of the seeds (irrigation) is set at a certain flow of nutrient solution (water) (e.g., 3.3 L min−1), it poses a risk of leaching the seeds. Therefore, in further studies, the hypothesis that the slope angle of the growing trays could be lower at the beginning of seed germination and could automatically increase after a certain stage of plant growth should be evaluated.Another study could investigate changing the flow rate of the nutrient solution for seed irrigation, namely, that at the beginning of the germination of wheat seeds, the slope angle (α) of the trays should (could) be high (i.e., 6.5% or 8.0%), but the nutrient solution flow rate (l min−1) could be lower. With the growth of seed root systems where there is no risk of seed leaching, this flow rate could be increased. Thus, hydroponically grown wheat fodder for feed should (could) be automated by changing the tray slope angle and/or nutrient solution flow rate for seed irrigation.
- Our study showed that plant yield was not uniform over the growing tray length. A significant decrease in yield was observed in the lower part of the tray length (about 150 mm). In the upper part of the tray (about 100 mm), the yield of wheat fodder was also slightly lower. This was probably because of the nutrient solution passing through the roots of the plants. The velocity of the solution was not uniform in the individual parts of the tray length. At the bottom, the roots of the plants were soaked. Therefore, in future studies, it would be appropriate to justify the shape of the bottom of the growing tray.
- A more detailed assessment of the environmental impact of using a hydroponic system in the production of fresh fodder should be conducted using a life-cycle assessment.
Author Contributions
Funding
Conflicts of Interest
References
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, H.; Roser, M. Meat and Dairy Production. Available online: https://ourworldindata.org/meat-production?fbclid=IwAR2I4y82fsZxHORHLWnsxcoeVKc9mSnMSURqynKD9AMtmttZ54a0GjXSYRU#citation (accessed on 7 January 2020).
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar] [CrossRef]
- Farghaly, M.M.; Abdullah, M.A.; Youssef, I.M.; Abdel-Rahim, I.R.; Abouelezz, K. Effect of feeding hydroponic barley sprouts to sheep on feed intake, nutrient digestibility, nitrogen retention, rumen fermentation and ruminal enzymes activity. Livest. Sci. 2019, 228, 31–37. [Google Scholar] [CrossRef]
- Van Kernebeek, H.R.; Oosting, S.J.; Van Ittersum, M.K.; Bikker, P.; De Boer, I.J. Saving land to feed a growing population: Consequences for consumption of crop and livestock products. Int. J. Life Cycle Assess. 2016, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Sardare, M.D.; Admane, S.V. A review on plant without soil-hydroponics. Int. J. Res. Eng. Technol. 2013, 2, 299–304. [Google Scholar] [CrossRef]
- Pierce, F.J.; Lal, R. Monitoring the impact of soil erosion on crop productivity. In Soil Erosion Research Methods; Routledge: Abingdon, UK, 2017; pp. 235–263. [Google Scholar] [CrossRef]
- Girma, F.; Gebremariam, B. Review on hydroponic feed value to livestock production. J. Sci. Innov. Res. 2018, 7, 106–109. [Google Scholar]
- Adebiyi, O.A.; Adeola, A.T.; Osinowo, O.A.; Brown, D.; Ng’ambi, J.W. Effects of feeding hydroponics maize fodder on performance and nutrient digestibility of weaned pigs. Appl. Ecol. Environ. Res. 2018, 16, 2415–2422. [Google Scholar] [CrossRef]
- Gebremedhin, W.K. Nutritional benefit and economic value of feeding hydroponically grown maize and barley fodder for Konkan Kanyal goats. IOSR. J. Agric. Vet. Sci. 2015, 8, 24–30. [Google Scholar]
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Soares, T.M.; Duarte, S.N.; De França, Ê.F.; Mélo, R.F.; De Andrade Jorge, C.; De Oliveira, Á.S. Experimental structure for evaluation of brackish water use in lettuce hydroponic production. Irriga 2009, 14, 102–114. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Lobillo-Eguíbar, J.; Fernández-Cañero, R.; Fernández-Cabanás, V.M. Suitability and optimization of FAO’s small-scale aquaponics systems for joint production of lettuce (Lactuca sativa) and fish (Carassius auratus). Aquac. Eng. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Hussein, T.H.A.; Abd El-Shafea, Y.M.; El-Behairy, U.A.A.; Abdallah, M.M.F. Effect of soaking and sprouting using saline water on chemical composition of wheat grains. Arab Univ. J. Agric. Sci. 2019, 27, 707–715. [Google Scholar] [CrossRef]
- Eyzaguirre, R.Z.; Nienaltowska, K.; De Jong, L.E.; Hasenack, B.B.; Nout, M.R. Effect of food processing of pearl millet (Pennisetum glaucum) IKMP-5 on the level of phenolics, phytate, iron and zinc. J. Sci. Food Agric. 2006, 86, 1391–1398. [Google Scholar] [CrossRef]
- Kalpanadevi, V.; Mohan, V.R. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculata (L.) Walp subsp. unguiculata. LWT Food Sci. Technol. 2013, 51, 455–461. [Google Scholar] [CrossRef]
- Yang, F.; Basu, T.K.; Ooraikul, B. Studies on germination conditions and antioxidant contents of wheat grain. Int. J. Food Sci. Nutr. 2001, 52, 319–330. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Delcour, J.A. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Ortiz, F.A.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Mora-Escobedo, R.; Rojas-León, A.; Rodríguez-Marín, M.L.; Román-Gutiérrez, A.D. Enzyme activity during germination of different cereals: A review. Food Rev. Int. 2019, 35, 177–200. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Al-Hashimi, M. Green fodder production and water use efficiency of some forage crops under hydroponic conditions. ISRN Agron. 2011, 2012, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Snow, A.M.; Ghaly, A.E.; Snow, A. A comparative assessment of hydroponically grown cereal crops for the purification of aquaculture wastewater and the production of fish feed. Am. J. Agric. Biol. Sci. 2008, 3, 364–378. [Google Scholar] [CrossRef]
- Guerrero-Cervantes, M.; Cerrillo-Soto, M.A.; Plascencia, A.; Salem, A.Z.M.; Estrada-Angulo, A.; Rios-Rincón, F.G.; Abdalla, A.L. Productive and reproductive performance and metabolic profiles of ewes supplemented with hydroponically grown green wheat (Triticum aestivum L.). Anim. Feed Sci. Technol. 2016, 221, 206–214. [Google Scholar] [CrossRef]
- Kide, W.; Desai, B.; Kumar, S. Nutritional improvement and economic value of hydroponically sprouted maize fodder. Life Sci. Int. Res. J. 2015, 2, 76–79. [Google Scholar]
- Fazaeli, H.; Golmohammadi, H.A.; Shoayee, A.A.; Montajebi, N.; Mosharraf, S. Performance of feedlot calves fed hydroponics fodder barley. J. Agric. Sci. Technol. 2011, 13, 367–375. [Google Scholar]
- Al Ajmi, A.; Salih, A.A.; Kadim, I.; Othman, Y. Yield and water use efficiency of barley fodder produced under hydroponic system in GCC countries using tertiary treated sewage effluents. J. Phytol. 2009, 1, 342–348. [Google Scholar]
- Wang, Q.; Zhao, H.; Xu, L.; Wang, Y. Uptake and translocation of organophosphate flame retardants (OPFRs) by hydroponically grown wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2019, 174, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Tayade, R.G.; Chavan, S.J. Development and performance of pipe framed hydroponic structure for fodder crop: A review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 341–350. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Grigas, A.; Kemzūraitė, A.; Steponavičius, D. Hydroponic devices for green fodder production: A review. In Proceedings of the International Scientific Conference “Rural Development”, Kaunas, Lithuania, 26–28 September 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Alinaitwe, J.; Nalule, A.S.; Okello, S.; Nalubwama, S.; Galukande, E. Nutritive and economic value of hydroponic barley fodder in kuroiler chicken diets. IOSR J. Agric. Vet. Sci. 2018, 12, 76–83. [Google Scholar]
- Rajesh, J.; Sivakumar, S.D.; Babu, C.; Srithran, N. Performance of different crops under hydroponics fodder production system. Madras Agric. J. 2018, 105, 50–55. [Google Scholar]
- Peckenpaugh, D. Hydroponic Solutions: Hydroponic Growing Tips; New Moon Publishing, Inc.: Corvallis, OR, USA, 2004; Volume 1. [Google Scholar]
- Morgan, L. Hydroponic Lettuce Production: A Comprehensive, Practical and Scientific Guide to Commercial Hydroponic Lettuce Production; Casper Publications: New South Wales, Australia, 1999. [Google Scholar]
- López-Pozos, R.; Martínez-Gutiérrez, G.A.; Pérez-Pacheco, R.; Urrestarazu, M. The effects of slope and channel nutrient solution gap number on the yield of tomato crops by a nutrient film technique system under a warm climate. HortScience 2011, 46, 727–729. [Google Scholar] [CrossRef] [Green Version]
- Pant, T.; Agarwal, A.; Bhoj, A.S.; Joshi, R.P.; Prakash, O.; Dwivedi, S.K. Vegetable cultivation under hydroponics in Himalayas: Challenges and opportunities. Def. Life Sci. J. 2018, 3, 111–119. [Google Scholar] [CrossRef]
- Kleiber, T.; Starzyk, J.; Bosiacki, M. Effect of nutrient solution, effective microorganisms (EM-A), and assimilation illumination of plants on the induction of the growth of lettuce (Lactuca sativa L.) in hydroponic cultivation. Acta Agrobot. 2013, 66, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Harun, A.N.; Ani, N.N.; Ahmad, R.; Azmi, N.S. Red and Blue LED with Pulse Lighting Control Treatment for Brassica Chinensis in Indoor Farming. In Proceedings of the IEEE Conference on Open Systems (ICOS), Kuching, Sarawak, Malaysia, 2–4 December 2013; pp. 231–236. [Google Scholar] [CrossRef]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Morgan, J.V.; Hunter, R.R.; O’Haire, R. Limiting Factors in Hydroponic Barley Grass Production. In Proceedings of the 8th International Congress on Soilless Culture, Rustenburg, South Africa, 2–9 October 1992; pp. 241–261. [Google Scholar]
- Koneva, M.S.; Rudenko, O.V.; Usatikov, S.V.; Bugayets, N.A.; Tamova, M.Y.; Fedorova, M.A. Optimization of soaking stage in technological process of wheat germination by hydroponic method when objective function is defined implicitly. J. Phys. Conf. Ser. 2018, 1015, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.; De Oliveira, L.F. Raman spectra of carotenoids in natural products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Czyczyło-Mysza, I.; Tyrka, M.; Marcińska, I.; Skrzypek, E.; Karbarz, M.; Dziurka, M.; Quarrie, S.A. Quantitative trait loci for leaf chlorophyll fluorescence parameters, chlorophyll and carotenoid contents in relation to biomass and yield in bread wheat and their chromosome deletion bin assignments. Mol. Breed. 2013, 32, 189–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiokias, S.; Proestos, C.; Varzakas, T. A review of the structure, biosynthesis, absorption of carotenoids-analysis and properties of their common natural extracts. Curr. Res. Nutr. Food Sci. J. 2016, 4, 25–37. [Google Scholar] [CrossRef]
- Olsson, U.; Engstrand, U.; Rupšys, P. Statistiniai Metodai SAS ir MINITAB (Statistical Methods using SAS and MINITAB); LŽŪU Leidybos Centras: Akademija, Lithuania, 2000. [Google Scholar]
- Wibowo, S. Aplikasi hidroponik NFT pada budidaya Pakcoy (Brassica rapa chinensis). (Application of NFT hydroponic on cultivation of Pakcoy (Brassica rapa chinensis)). J. Penelit. Pertan. Terap. 2017, 13, 159–167. [Google Scholar]
- Bertholdsson, N.O. Screening for barley waterlogging tolerance in nordic barley cultivars (Hordeum vulgare L.) using chlorophyll fluorescence on hydroponically-grown plants. Agronomy 2013, 3, 376–390. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Maekawa, T. Induction of homogeneous rooting control in liquid cultured brown rice using hypoxic conditions. Seed Sci. Technol. 2000, 28, 367–379. [Google Scholar]
- Que, F.; Wang, G.L.; Feng, K.; Xu, Z.S.; Wang, F.; Xiong, A.S. Hypoxia enhances lignification and affects the anatomical structure in hydroponic cultivation of carrot taproot. Plant. Cell Rep. 2018, 37, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.P.S.; Wadhwa, M.; Makkar, H.P.S. Hydroponic fodder production: A critical assessment. Broadening Horiz. 2017, 48, 1–10. [Google Scholar]
- Karaşahin, M. Topraksız ortamda yetiştirilen bazı tahılların çim suyu verim ve besin değerleri (Grass juice yield and nutritional values of some cereals in soilless cultur). Iğdır Üniversitesi Fen Bilimleri Enstitüsü Dergisi 2015, 5, 57–64. [Google Scholar]
- Ali, H.S.; Miah, A.G.; Sabuz, S.H.; Asaduzzaman, M.; Salma, U. Dietary effects of hydroponic wheat sprouted fodder on growth performance of turkey. Res. Agric. Livest. Fish. 2019, 6, 101–110. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 3, 1951–1956. [Google Scholar] [CrossRef]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Agius, A.; Pastorelli, G.; Attard, E. Cows fed hydroponic fodder and conventional diet: Effects on milk quality. Arch. Anim. Breed. 2019, 62, 517–525. [Google Scholar] [CrossRef]










| Name, Type, and Number of Measuring Instrument and Equipment | Range of Measurement | Measurement Accuracy |
|---|---|---|
| Thermometer—Sencor SWS 1500 RD | 0–50 °C | ±1 °C (from 0 °C to 40 °C) |
| Room humidity meter—Sencor SWS 1500 RD | 20–95% | ±5% (from 40% to 80%) |
| Light flux meter—LX1010B | 0–50.000 Lx | ±5% (from 0 °C to 50 °C) |
| Digital spirit level—Bosch DNM 60L | 0–90° | ±0.05° |
| Drying and heating chamber—Binder FP400 | 5–300 °C | ±2.5 °C (at 150 °C) |
| Electrical conductivity detector—ADWA AD-204 | 0–19.99 mS cm−1 | <1% |
| Electronic weighing scales—METTLER TOLEDO SB 16001 | 0–16 kg | ±0.1 g |
| Spectrophotometer—LABOMED UVD-3200 | 190–1100 nm | ±0.3 nm |
| Lighting flow controller—TC420 | 0–24 V | — |
| Costs, EUR | Unit | Quantity | Unit Price, EUR with VAT | Price, EUR | Share of Total, % |
|---|---|---|---|---|---|
| Grains for sowing | kg | 185.40 | 0.16 | 29.66 | 34.83 |
| Heating | kWh | 46.70 | 0.15 | 7.01 | 8.22 |
| Electricity * | kWh | 8.40 | 0.15 | 1.26 | 1.48 |
| Water | m3 | 5.59 | 2.50 | 13.98 | 16.41 |
| Fertilizers | |||||
| Calcium nitrate | kg | 5.40 | 1.50 | 8.10 | 9.51 |
| Magnesium sulfate | kg | 1.50 | 1.70 | 2.55 | 2.99 |
| Potassium nitrate | kg | 8.10 | 2.20 | 17.82 | 20.92 |
| Universol violet complex fertilizer | kg | 1.50 | 3.20 | 4.80 | 5.64 |
| 33.27 | 39.06 | ||||
| Cost of cultivation of 1000 kg hydroponic feed, EUR | 85.18 | 100 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigas, A.; Kemzūraitė, A.; Steponavičius, D.; Steponavičienė, A.; Domeika, R. Impact of Slope of Growing Trays on Productivity of Wheat Green Fodder by a Nutrient Film Technique System. Water 2020, 12, 3009. https://doi.org/10.3390/w12113009
Grigas A, Kemzūraitė A, Steponavičius D, Steponavičienė A, Domeika R. Impact of Slope of Growing Trays on Productivity of Wheat Green Fodder by a Nutrient Film Technique System. Water. 2020; 12(11):3009. https://doi.org/10.3390/w12113009
Chicago/Turabian StyleGrigas, Andrius, Aurelija Kemzūraitė, Dainius Steponavičius, Aušra Steponavičienė, and Rolandas Domeika. 2020. "Impact of Slope of Growing Trays on Productivity of Wheat Green Fodder by a Nutrient Film Technique System" Water 12, no. 11: 3009. https://doi.org/10.3390/w12113009
APA StyleGrigas, A., Kemzūraitė, A., Steponavičius, D., Steponavičienė, A., & Domeika, R. (2020). Impact of Slope of Growing Trays on Productivity of Wheat Green Fodder by a Nutrient Film Technique System. Water, 12(11), 3009. https://doi.org/10.3390/w12113009