Impacts of Chosen Parameters on Fe-Dependent Nitrate Reduction in Anammox Consortia: Performance and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Wastewater
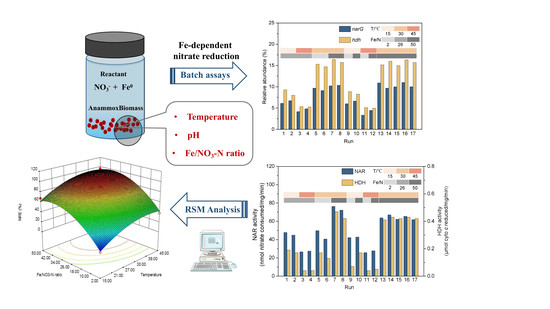
2.2. Batch Assays Procedure
2.3. Determination of Enzymes Activity and Functional Genes Expression
2.4. Response Surface Method (RSM) for Evaluating Individual and Interactive Effects of Parameters on Nitrate and Nitrogen Removal
2.5. Chemical Analysis
3. Results
3.1. Performance, Enzyme Activity, and Genes Expression with Different Parameters
3.2. Statistical Analysis
3.3. Discrepant Effects of Temperature, pH, and Fe/NO3-N Ratio on NRE and TNRE
3.3.1. Individual Effects
3.3.2. Interactive Effects
3.4. Model Optimization and Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Straub, K.L.; Benz, M.; Schink, B.; Widde, F. Anaerobic nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microb. 1996, 62, 1458–1460. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Lien, H.L.; Koel, B.E.; Zhang, W. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, P.; Wang, R.; Li, W.; Lu, H.; Zhang, J. Nitrate-dependent anaerobic ferrous oxidation (NAFO) by denitrifying bacteria: A perspective autotrophic nitrogen pollution control technology. Chemosphere 2014, 117, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Huang, T.; Guan, X. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef]
- Strous, M.; Pelletier, E.; Mangenot, S.; Rattei, T.; Lehner, A.; Taylor, M.W.; Horn, M.; Daims, H.; Bartol-Mavel, D.; Wincker, P.; et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 2006, 440, 790–794. [Google Scholar] [CrossRef]
- Oshiki, M.; Ishii, S.; Yoshida, K.; Fujii, N.; Ishiguro, M.; Satoh, H. Nitrate-dependent ferrous iron oxidation by anaerobic ammonium oxidation (anammox) bacteria. Appl. Environ. Microb. 2013, 79, 4087–4093. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Zhang, W.; Song, G.; Huang, Y. Iron-dependent nitrate reduction by anammox consortia in continuous-flow reactors: A novel prospective scheme for autotrophic nitrogen removal. Sci. Total Environ. 2019, 692, 582–588. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Cema, G.; Ziembińska-Buczyńska, A. Influence of temperature and pH on the anammox process: A review and meta-analysis. Chemosphere 2017, 182, 203–214. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; Van Hullebusch, E.D.; Esposito, G. Fe(II)—Mediated autotrophic denitrification: A new bioprocess for iron bioprecipitation/biorecovery and simultaneous treatment of nitrate-containing wastewaters. Int. Biodeterior. Biodegr. 2016, 119, 631–648. [Google Scholar] [CrossRef]
- Weber, K.A.; Pollock, J.; Cole, K.A.; O’Connor, S.M.; Achenbach, L.A.; Coates, J.D. Anaerobic nitrate-dependent iron (II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microb. 2006, 72, 686–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Graff, A.A.; De Bruijn, P.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Chubukov, V.; Uhr, M.; Chat, L.L.; Kleijn, R.J.; Jules, M.; Link, H.; Aymerich, S.; Stelling, J.; Sauer, U. Transcriptional regulation is insufficient to explain substrate-induced flux changes in Bacillus subtilis. Mol. Syst. Biol. 2013, 9, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.B.; Yu, H.Q.; Harada, H.; Li, Y.Y. Optimization of anaerobic acidogenesis of an aquatic plant, Canna indica L., by rumen cultures. Water Res. 2007, 41, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.S.; Guo, Q.; Zhang, Z.Z.; Zhang, J.; Wang, H.Z.; Jin, R.C. Optimization of process performance in a granule-based anaerobic ammonium oxidation (anammox) upflow anaerobic sludge blanket (uasb) reactor. Bioresour. Technol. 2014, 170, 404–412. [Google Scholar] [CrossRef]
- Zinatizadeh, A.A.L.; Pirsaheb, M.; Bonakdari, H.; Younesi, H. Response surface analysis of effects of hydraulic retention time and influent feed concentration on performance of an UASFF bioreactor. Waste Manag. 2012, 30, 1798–1807. [Google Scholar] [CrossRef]
- Moon, C.; Singh, R.; Chaganti, S.R.; Lalman, J.A. Modeling sulfate removal by inhibited mesophilic mixed anaerobic communities using a statistical approach. Water Res. 2013, 47, 2341–2351. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Strous, M.; Kuenen, J.G.J.; Jetten, M.S.M. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microb. 1999, 65, 3248–3250. [Google Scholar] [CrossRef] [Green Version]
- Dosta, J.; Fernández, I.; Vázquez-Padín, J.R.; Mosquera-Corral, A.; Campos, J.L.; Mata-álvarez, J. Short-and long-term effects of temperature on the anammox process. J. Hazard. Mater. 2008, 154, 688–693. [Google Scholar] [CrossRef]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, Y.; Xue, Y.; Zhang, Y.; Li, Y. Relationship of heme c, nitrogen loading capacity and temperature in anammox reactor. Sci. Total Environ. 2019, 659, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Peng, Y.; Li, B.; Guo, J.; Yang, Q.; Wan, S. Biological removal of nitrogen from wastewater. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2008; pp. 159–195. [Google Scholar]
- Qiao, S.; Bi, Z.; Zhou, J.; Cheng, Y.; Zhang, J. Long term effects of divalent ferrous ion on the activity of anammox biomass. Bioresour. Technol. 2013, 142, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Qiao, S.; Zhou, J.; Tang, X.; Zhang, J. Fast start-up of anammox process with appropriate ferrous iron concentration. Bioresour. Technol. 2014, 170, 506–512. [Google Scholar] [CrossRef]
- Ferousi, C.; Lindhoud, S.; Baymann, F.; Kartal, B.; Jetten, M.S.M.; Reiman, J. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria. Curr. Opin. Chem. Biol. 2017, 37, 129–136. [Google Scholar] [CrossRef]
- You, G.; Wang, P.; Hou, J.; Wang, C.; Xu, Y.; Miao, L.; Lv, B.; Yang, Y.; Zhang, F. The use of zero-valent iron (ZVI)-microbe technology for wastewater treatment with special attention to the factors influencing performance: A critical review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 877–907. [Google Scholar] [CrossRef]
- Lavania, A.; Bose, P. Effect of metallic iron concentration on end-product distribution during metallic iron-assisted autotrophic denitrification. J. Environ. Eng. 2006, 132, 994–1000. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef]
- Straub, K.L.; Schönhuber, W.A.; Buchholz-Cleven, B.E.; Schink, B. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 2004, 21, 371–378. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, F.; Xu, X. Optimization of nitrate removal from wastewater with a low C/N ratio using solid-phase denitrification. Environ. Sci. Pollut. R. 2016, 23, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, Y.; Lv, L.; Ahmad, H.A.; Ni, S.; Ren, L.; Gui, Z.; Fang, X.; Qiao, Z.; Ding, S. Transformation of the zero valent iron dosage effect on anammox after long-term culture: From inhibition to promotion. Process Biochem. 2019, 78, 132–139. [Google Scholar] [CrossRef]






| Batch Test | pH | Temp (°C) | Fe/NO3-N Ratio | n-Fe Powder Dosage (g/L) | NRE (%) | TNRE (%) | ||
|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |||||
| 1 | 4 | 15 | 26 | 7.28 | 65.95 | 67.64 | 46.81 | 45.75 |
| 2 | 8 | 15 | 26 | 7.28 | 51.2 | 52.52 | 39.42 | 34.17 |
| 3 | 4 | 45 | 26 | 7.28 | 96.22 | 94.91 | 12.58 | 17.69 |
| 4 | 8 | 45 | 26 | 7.28 | 90.05 | 88.37 | 13.37 | 14.29 |
| 5 | 4 | 30 | 2 | 0.56 | 51.32 | 47.77 | 48.91 | 52.55 |
| 6 | 8 | 30 | 2 | 0.56 | 45.27 | 42.10 | 39.32 | 37.15 |
| 7 | 4 | 30 | 50 | 14 | 91.51 | 94.72 | 81.82 | 73.86 |
| 8 | 8 | 30 | 50 | 14 | 85.11 | 88.72 | 78.03 | 74.27 |
| 9 | 6 | 15 | 2 | 0.56 | 9.00 | 10.86 | 8.96 | 6.31 |
| 10 | 6 | 15 | 50 | 14 | 67.78 | 63.03 | 38.54 | 47.41 |
| 11 | 6 | 45 | 2 | 0.56 | 38.01 | 42.80 | 3.21 | −5.78 |
| 12 | 6 | 45 | 50 | 14 | 96.02 | 94.20 | 9.08 | 11.56 |
| 13 | 6 | 30 | 26 | 7.28 | 90.5 | 88.54 | 71.48 | 71.82 |
| 14 | 6 | 30 | 26 | 7.28 | 88.07 | 88.54 | 69.81 | 71.82 |
| 15 | 6 | 30 | 26 | 7.28 | 87 | 88.54 | 70.5 | 71.82 |
| 16 | 6 | 30 | 26 | 7.28 | 91.09 | 88.54 | 78.03 | 71.82 |
| 17 | 6 | 30 | 26 | 7.28 | 86 | 88.54 | 69.57 | 71.82 |
| Term | Sum of Squares (S.S.) | Mean Square (M.S.) | F Value | p-Value (Prob > F) | ||||
|---|---|---|---|---|---|---|---|---|
| S.S.NRE | S.S.TNRE | M.S.NRE | S.S.TNRE | FNRE | FTNRE | pNRE | pTNRE | |
| Model | 10,688.23 | 12,401.79 | 1187.58 | 1377.98 | 65.56 | 22.45 | <0.0001 | 0.0002 |
| X1-pH | 235.12 | 112.35 | 235.12 | 112.35 | 12.98 | 1.83 | 0.0087 | 0.2182 |
| X2-Temperature | 1996.17 | 1139.79 | 1996.17 | 1139.79 | 110.19 | 18.57 | <0.0001 | 0.0035 |
| X3-Fe/NO3-N ratio | 5346.81 | 1713.17 | 5346.81 | 1713.17 | 295.16 | 27.91 | <0.0001 | 0.0011 |
| X1X2 | 18.40 | 16.73 | 18.40 | 16.73 | 1.02 | 0.27 | 0.3470 | 0.6178 |
| X1X3 | 23.28 | 62.41 | 23.28 | 62.41 | 1.29 | 1.02 | 0.2943 | 0.3469 |
| X2X3 | 0.15 | 140.54 | 0.15 | 140.54 | 0.008 | 2.29 | 0.3305 | 0.0740 |
| X12 | 0.19 | 0.58 | 0.19 | 0.58 | 0.010 | 0.009 | 0.9216 | 0.9256 |
| X22 | 699.42 | 8226.88 | 699.42 | 8226.88 | 38.61 | 134.01 | 0.0004 | <0.0001 |
| X32 | 2215.96 | 682.09 | 2215.96 | 682.09 | 122.33 | 11.11 | <0.0001 | 0.0125 |
| Test | pH | Temp. (°C) | Fe/NO3-N Ratio | NRE (%) | TNRE (%) | ||
|---|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | ||||
| 1 | 6.0 | 28 | 35 | 92.37 | 92.70 | 77.48 | 76.61 |
| 2 | 5.0 | 30 | 48 | 88.46 | 94.667 | 79.36 | 74.67 |
| 3 | 8.0 | 35 | 20 | 77.95 | 79.38 | 52.91 | 55.28 |
| 4 | 4.0 | 22 | 30 | 80.65 | 86.53 | 67.27 | 72.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Z.; Huang, Y.; Zhang, W.; Song, G. Impacts of Chosen Parameters on Fe-Dependent Nitrate Reduction in Anammox Consortia: Performance and Bioactivity. Water 2020, 12, 1379. https://doi.org/10.3390/w12051379
Bi Z, Huang Y, Zhang W, Song G. Impacts of Chosen Parameters on Fe-Dependent Nitrate Reduction in Anammox Consortia: Performance and Bioactivity. Water. 2020; 12(5):1379. https://doi.org/10.3390/w12051379
Chicago/Turabian StyleBi, Zhen, Yong Huang, Wenjing Zhang, and Ge Song. 2020. "Impacts of Chosen Parameters on Fe-Dependent Nitrate Reduction in Anammox Consortia: Performance and Bioactivity" Water 12, no. 5: 1379. https://doi.org/10.3390/w12051379
APA StyleBi, Z., Huang, Y., Zhang, W., & Song, G. (2020). Impacts of Chosen Parameters on Fe-Dependent Nitrate Reduction in Anammox Consortia: Performance and Bioactivity. Water, 12(5), 1379. https://doi.org/10.3390/w12051379