The Use of Zooplankton Metrics to Determine the Trophic Status and Ecological Potential: An Approach in a Large Mediterranean Watershed
Abstract
:1. Introduction
2. Materials and Methods
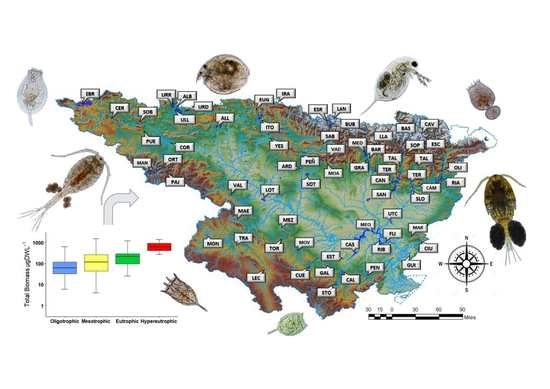
2.1. Study Site
2.2. Environmental Variables
2.3. Trophic State and Ecological Potential
2.4. Zooplankton Samples
2.5. Zooplankton Metrics
2.6. Statistical Analysis
3. Results
3.1. Environmental Data, Trophic State, and Ecological Potential
3.2. Zooplankton Community Description
3.3. Statistical Interpretation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code | Reservoir | Average Trophic Status | Average Ecological Potential | Location |
|---|---|---|---|---|
| ALB | Albiña | Oligo–Mesotrophic | Moderate | Pais Vasco |
| ALL | Alloz | Oligotrophic | Good–Moderate | Navarra |
| ARD | Ardisa | Eutrophic | Moderate | Aragón |
| BAL | Balaguer | Mesotrophic | Good or superior | Cataluña |
| BAR | Barasona | Oligotrophic | Good or superior | Aragón |
| BAS | Baserca | Oligotrophic | Good or superior | Aragón |
| BUB | Búbal | Oligotrophic | Good or superior | Aragón |
| CAL | Calanda | Oligotrophic | Good–Moderate | Aragón |
| CAM | Camarasa | Oligotrophic | Good or superior | Cataluña |
| CAN | Canelles | Oligotrophic | Good or superior | Aragón |
| CAS | Caspe | Mesotrophic | Moderate | Aragón |
| CAV | Cavallers | Oligotrophic | Good or superior | Cataluña |
| CER | Cereceda | Eutrophic | Moderate | Castilla y León |
| CIU | Çiurana | Oligotrophic | Good or superior | Cataluña |
| COR | El Cortijo | Eutrophic | Moderate | La Rioja |
| CUE | Foradada | Mesotrophic | Moderate | Aragón |
| EBR | Ebro | Oligo–Mesotrophic | Moderate | Cantabria |
| ESC | Escales | Oligotrophic | Good or superior | Aragón |
| ESR | Escarra | Oligotrophic | Good or superior | Aragón |
| EST | Alcañiz | Mesotrophic | Good–Moderate | Aragón |
| EUG | Eugui | Oligotrophic | Good or superior | Navarra |
| FLI | Flix | Mesotrophic | Moderate | Cataluña |
| GAL | Gallipuén | Mesotrophic | Moderate | Aragón |
| GRA | El Grado | Oligotrophic | Good or superior | Aragón |
| GUI | Guiamets | Mesotrophic | Moderate | Cataluña |
| IRA | Irabia | Oligotrophic | Moderate | Navarra |
| ITO | Itoiz | Oligotrophic | Good or superior | Navarra |
| LAN | Lanuza | Oligotrophic | Good or superior | Aragón |
| LEC | Lechago | Oligo–Mesotrophic | Moderate | Aragón |
| LLA | Llauset | Oligotrophic | Good or superior | Aragón |
| LOT | La Loteta | Meso–Eutrophic | Moderate | Aragón |
| MAE | Maidevera | Mesotrophic | Moderate | Aragón |
| MAN | Mansilla | Oligotrophic | Good–Moderate | La Rioja |
| MAR | Margalef | Mesotrophic | Moderate | Cataluña |
| MED | Mediano | Oligotrophic | Good or superior | Aragón |
| MEQ | Mequinenza | Oligo–Mesotrophic | Moderate | Aragón |
| MEZ | Mezalocha | Meso–Eutrophic | Moderate | Aragón |
| MOA | Montearagon | Oligotrophic | Good–Moderate | Aragón |
| MON | Vicarías | Mesotrophic | Moderate | Castilla y León |
| MOV | Moneva | Meso–Eutrophic | Moderate | Aragón |
| OLI | Oliana | Mesotrophic | Moderate | Cataluña |
| ORT | Ortigosa | Oligotrophic | Good or superior | La Rioja |
| PAJ | Pajares | Oligotrophic | Good or superior | La Rioja |
| PEÑ | La Peña | Mesotrophic | Moderate | Aragón |
| PEN | Pena | Oligotrophic | Good or superior | Aragón |
| PUE | Puentelarra | Mesotrophic | Moderate | Castilla y León |
| RIA | Rialb | Mesotrophic | Moderate | Cataluña |
| RIB | Ribarroja | Eutrophic | Moderate | Cataluña |
| SAB | Sabiñanigo | Oligotrophic | Good or superior | Aragón |
| SAN | Santa Ana | Oligotrophic | Good or superior | Cataluña |
| SLO | San Lorenzo | Mesotrophic | Good or superior | Cataluña |
| SOB | Sobrón | Meso–Eutrophic | Moderate | Castilla y León |
| SOP | Sopeira | Oligotrophic | Good or superior | Aragón |
| SOT | Sotonera | Mesotrophic | Moderate | Aragón |
| STO | Santolea | Oligotrophic | Good or superior | Aragón |
| TAL | Talarn | Oligo–Mesotrophic | Good or superior | Cataluña |
| TER | Terradets | Mesotrophic | Moderate | Cataluña |
| TOR | Las Torcas | Oligo–Mesotrophic | Good or superior | Aragón |
| TRA | Tranquera | Mesotrophic | Moderate | Aragón |
| ULL | Ullivari | Oligo–Mesotrophic | Good–Moderate | Pais Vasco |
| URD | Urdalur | Oligotrophic | Good or superior | Navarra |
| URR | Urrunaga | Oligo–Mesotrophic | Moderate | Pais Vasco |
| UTC | Utexa seca | Eutrophic | Moderate | Cataluña |
| VAD | Vadiello | Oligo–Mesotrophic | Good or superior | Aragón |
| VAL | Val | Eutrophic | Moderate | Aragón |
| YES | Yesa | Oligotrophic | Good–Moderate | Navarra |
Appendix B
| Rotifera | |||
| Class Bdelloidea | C. unicornis | L. puriformis | P. triloba |
| Bdelloids | Conochilus sp. | L. stenroosi | Proales sp. |
| Class Monogononta | Dicranophorus sp. | L. stichaea | Ptygura sp. |
| Anuraeopsis fissa | Encentrum sp. | L. tenuiseta | Squatinella rostrum |
| Ascomorpha ecaudis | Eosphora sp. | Lecane sp. | Synchaeta grandis |
| A. ovalis | Euchlanis dilatata | Lepadella acuminata | S. kitina |
| A. saltans | Filinia longiseta | L. ovalis | S. longipes |
| Ascomorpha sp. | F. terminalis | L. patella | S. oblonga |
| Asplanchna girodi | Gastropus stylifer | L. rhomboides | S. pectinata |
| A. priodonta | Hexarthra fennica | Lophocaris salpina | S. stylata |
| A. sieboldi | H. intermedia | L. oxysternon | S. tremula |
| Asplanchna sp. | H. mira | Macrochaetus subquadratus | Synchaeta sp. |
| Brachionus angularis | H. oxyuris | Monommata appendiculata | Testudinella incisa |
| B. bidentata | Hexarthra sp. | Mytilina mucronata | T. mucronata |
| B. calyciflorus | Kellicottia longispina | Notholca acuminata | T. patina |
| B. dimidiatus | Keratella cochlearis | N. squamula | Trichocerca cylindrica |
| B. havanaensis | K. cochlearis tecta | Notommata allantois | T. gracilis |
| B. plicatilis | K. hiemalis | N. copeus | T. inermis |
| B. quadridentatus | K. quadrata | Ploesoma hudsoni | T. insignis |
| B. urceolaris | K. tropica | P. lenticulare | T. pusilla |
| Cephalodella gibba | Lecane aculeata | P. truncatum | T. similis |
| C. stenroosi | L. bulla | Polyarthra dolichoptera | T. tenuinor |
| Cephalodella sp. | L. clara | P. euryptera | T. tigris |
| Collotheca pelagica | L. closterocerca | P. longiremis | Trichocerca sp. |
| Collotheca sp. | L. cornuta | P. luminosa | Trichotria pocillum |
| Colurella colurus | L. flexilis | P. major | T. tetractis |
| C. obtusa | L. furcata | P. minor | Tripleuchlanis plicata |
| C. uncinata | L. inermis | Polyarthra vulgaris | |
| Conochilus dossuarius | L. luna | Polyarthra sp. | |
| C. natans | L. lunaris | Phompolyx sulcata | |
| Crustacea | |||
| Suborder Cladocera | |||
| Alona affinis | D. parvula | Copepoda | Order Harpacticoida |
| A. guttata | D. pulicaria | Order Cyclopoida | Harpacticoids |
| A. quadrangularis | Daphnia rosea | Acanthocyclops americanus | |
| A. rectangula | Diaphanosoma brachyurum | A. robustus | Order Poecilostomatoida |
| Alona sp. | D. lacustris | Cyclops abyssorum | Ergasilus sieboldi |
| Alonella exigua | D. mongolianum | C. lacustris | Neoergasilus japonicus |
| A. nana | Diaphanosoma sp. | C. vicinus | |
| Bosmina longirostris | Holopedium gibberum | Cyclops sp. | Mollusca |
| Ceriodaphnia dubia | Ilyocryptus sordidus | Eucyclops serrulatus | Class Bivalvia |
| C. laticaudata | Leydigia acanthocercoides | Eucyclops sp. | Dreissena polymorpha |
| C. pulchella | L. leydigi | Macrocyclops albidus | |
| C. quadrangula | L. quadrangularis | Thermocyclops dybowskii | |
| Chydorus sphaericus | Macrothrix hirsuticornis | Tropocyclops prasinus | |
| Daphnia cucullata | M. laticornis | ||
| D. curvirostris | Moina micrura | Order Calanoida | |
| D. galeata | Oxyurella tenuicaudis | Copidodiaptomus numidicus | |
| D. longispina | Phrixura leei | Eudiaptomus vulgaris | |
| D. magna | Sida crystalina | Neolovenula alluaudi |
References
- Grantham, T.E.; Figueroa, R.; Prat, N. Water management in mediterranean river basins: A comparison of management frameworks, physical impacts, and ecological responses. Hydrobiologia 2013, 719, 451–482. [Google Scholar] [CrossRef]
- Schindler, D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B 2012, 279, 4322–4333. [Google Scholar] [CrossRef] [Green Version]
- Moss, B. Allied attack: Climate change and eutrophication. Inland Waters 2012, 1, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Kosten, S.; Huszar, V.M.; Bécares, E.; Costa, S.; van Donk, E.; Hansson, L.; Lurling, M.F.L.L.W. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Directive, W.F. Directive 2000/60/EC of the European parliament and of the council of establishing a framework for community action in the field of water policy. Off. J. Eur. Communities 2000, 327, 1–72. [Google Scholar]
- Moss, B. Shallow lakes, the water framework directive and life. What should it all be about? Hydrobiologia 2007, 584, 381–394. [Google Scholar] [CrossRef]
- Caroni, R.; Irvine, K. The potential of zooplankton communities for ecological assessment of lakes: Redundant concept or political oversight? In Biology and Environment: Proceedings of the Royal Irish Academy; JSTOR: New York, NY, USA, 2010; pp. 35–53. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Torben, L.; Lauridsen, T.L.; Søndergaard, M.; Sazer, C.; et al. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Haberman, J.; Haldna, M. Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: Long term study of Lake Vőrtsjärv. J. Limnol. 2014, 73, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Lampert, W.; Sommer, U. Limnoecology. In The Ecology of Lakes and Streams; Oxford University Press: New York, NY, USA, 1997; p. 382. [Google Scholar]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Sommer, U. Trophic cascades in marine and freshwater plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Rossetti, G. Fifty years after the homage to santa rosalia: Old and new paradigms on biodiversity in aquatic ecosystems. In Santa Rosalia 50 Years on Developments in Hydrobiology; Springer: Cham, Switzerland, 2010; Volume 213, p. 246. [Google Scholar]
- Dodson, S.I.; Newman, A.L.; Will-Wolf, S.; Alexander, M.L.; Woodford, M.P.; Van Egeren, S. The relationship between zooplankton community structure and lake characteristics in temperate lakes (Northern Wisconsin, USA). J. Plankton Res. 2009, 31, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Bonecker, C.; Simões, N.; Minte-Vera, C.; Lansac-Tôha, F.; Velho, L.; Agostinho, A. Temporal changes in zooplankton species diversity in response to environmental changes in an alluvial valley. Limnologica 2013, 43, 114–121. [Google Scholar] [CrossRef]
- Anas, M.U.; Scott, K.A.; Wissel, B. Suitability of presence vs. absence indicator species to characterize stress gradients: Lessons from zooplankton species of boreal lakes. Ecol. Ind. 2013, 30, 90–99. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Špoljar, M.; Zhang, C.; Pronin, M. Zooplankton functional traits as a tool to assess latitudinal variation in the northern-southern temperate European regions during spring and autumn seasons. Ecol. Ind. 2020, 117, 106629. [Google Scholar] [CrossRef]
- Čeirāns, A. Zooplankton indicators of trophy in Latvian lakes. Acta Univ. Latv. Biol. 2007, 723, 61–69. [Google Scholar]
- Brito, S.L.; Maia-Barbosa, P.M.; Pinto-Coelho, R.M. Zooplankton as an indicator of trophic conditions in two large reservoirs in Brazil. Lakes Reserv. Res. Manag. 2011, 16, 253–264. [Google Scholar] [CrossRef]
- Tasevska, O.; Špoljar, M.; Gušeska, D.; Kostoski, G.; Patcheva, S.; Veljanoska, S.E. Zooplankton in ancient and oligotrophic Lake Ohrid (Europe) in association with environmental variables. Croat. J. Fish. 2017, 75, 95–103. [Google Scholar] [CrossRef]
- Pociecha, A.; Bielańska-Grajner, I.; Kuciel, H.; Wojtal, A.Z. Is zooplankton an indicator of the water trophic level in dam reservoirs? Oceanol. Hydrobiol. Stud. 2018, 47, 288–295. [Google Scholar] [CrossRef]
- Duggan, C.; Green, J.D.; Thomasson, K. Do rotifers have potential as bioindicators of lake trophic state? Int. Ver. Theor. Angew. Limnol. Verh. 2001, 27, 3497–3502. [Google Scholar] [CrossRef]
- Boix, D.; Gascón, S.; Sala, J.; Martinoy, M.; Gifre, J.; Quintana, X.D. A new index of water quality assessment in Mediterranean wetlands based on crustacean and insect assemblages: The case of Catalunya (NE Iberian Peninsula). Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 635–651. [Google Scholar] [CrossRef]
- Cheng, G.; Dalton, C.; Taylor, D. Cladocera as indicators of trophic state in Irish lakes. J. Paleolimnol. 2010, 44, 465–481. [Google Scholar] [CrossRef]
- Pinto-Coelho, R.M.; Pinel-Alloul, B.; Méthot, G.; Havens, K.E. Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: Variations with trophic status. Can. J. Fish. Aquat. Sci. 2005, 61, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Montagud, D.; Soria, J.M.; Soria-Perpiña, X.; Vicente, E. A comparative study of four indexes based on zooplankton as trophic state indicators in reservoirs. Limnetica 2019, 38, 291–302. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Soria, J.M.; Vicente, E. Can zooplankton species be used as indicators of trophic status and ecological potential of reservoirs? Aquat. Ecol. 2021, in press. [Google Scholar] [CrossRef]
- Almeida, R.; Formigo, N.E.; Sousa-Pinto, I.; Antunes, S.C. Contribution of zooplankton as a biological element in the assessment of reservoir water quality. Limnetica 2020, 39, 245–261. [Google Scholar] [CrossRef]
- García-Chicote, J.; Armengol, X.; Rojo, C. Zooplankton species as indicators of trophic state in reservoirs from Mediterranean river basins. Inland Waters 2019, 9, 113–123. [Google Scholar] [CrossRef]
- May, L.; O’Hare, M. Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK. Hydrobiologia 2005, 546, 397–404. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Ejsmont-Karabin, J.; Karabin, A. The suitability of zooplankton as lake ecosystem indicators: Crustacean trophic state index. Pol. J. Ecol. 2013, 61, 561–573. [Google Scholar]
- Ochocka, A.; Pasztaleniec, A. Sensitivity of plankton indices to lake trophic conditions. Environ. Monit. Assess. 2016, 188, 662. [Google Scholar] [CrossRef] [Green Version]
- Kehayias, G.; Doulka, E. Trophic State Evaluation of a Large Mediterranean Lake Utilizing Abiotic and Biotic Elements. J. Environ. Prot. 2014, 5, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Trophic state assessment based on zooplankton communities in Mediterranean lakes. Hydrobiologia 2019, 844, 83–103. [Google Scholar] [CrossRef]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. The neglected zooplankton communities as indicators of ecological water quality of Mediterranean lakes. Limnetica 2021, 40, 359–373. [Google Scholar] [CrossRef]
- Geraldes, A.M.; Pasupuleti, R. Zooplankton: A valuable environmental indicator tool in reservoir ecological management? Asian J. Environ. Ecol. 2016, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- García-Chicote, J.; Armengol, X.; Rojo, C. Zooplankton abundance: A neglected key element in the evaluation of reservoir water quality. Limnologica 2018, 69, 46–54. [Google Scholar] [CrossRef]
- Vicente, E.; Hoyos, C.; Sánchez, P.; Cambra, J. Protocolo de Muestreo y Análisis para Fitoplancton; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, España, 2005.
- Shoaf, W.T.; Lium, B.W. Improved extraction of chlorophyll a and b from algae using dimethyl sulphoxide. Limnol. Oceanogr. 1976, 21, 926–928. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Catalan, J.; Ventura, M. Desenvolupament d´un Índex Integral de Qualitat Ecológica Iregionalizació Ambiental dels Sistemes Lacustres de Catalunya; Agència Catalana de l´Aigua: Barcelona, España, 2003.
- Confederación Hidrográfica Del Ebro. Establecimiento de una Metodología para el Seguimiento del Potencial vs. Estado Trófico de la Cuenca del Ebro; Confederación Hidrográfica Del Ebro: Zaragoza, España, 2016; p. 212. [Google Scholar]
- Miracle, M.R.; Vicente, E. Vertical distribution and rotifer concentrations in the chemocline of meromictic lakes. Hydrobiologia 1983, 104, 259–267. [Google Scholar] [CrossRef]
- Koste, W. Rotatoria. In Die Rädertiere Mitteleuropas; Borntraeger: Berlin, Germany, 1978; p. 672. [Google Scholar]
- Nogrady, T.; Segers, H. Rotifera 6: Asplanchnidae, Gastropodidae, Linfiidae, Microcodidae, Synchaetidae, Trochosphaeridae and Filinia. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H., Nogrady, T., Eds.; SPB Academic Publishing BV: Amsterdam, The Netherlands, 2002; p. 264. [Google Scholar]
- Ruttner-Kolisko, A. Plankton Rotifers: Biology and Taxonomy; Schweizerbart: Stuttgart, Germany, 1974; p. 146. [Google Scholar]
- Alonso, M. Crustacea, Branchiopoda. In Serie Fauna Ibérica; Museo Nacional De Ciencias Naturales CSIC: Madrid, Spain, 1996; Volume 7, p. 486. [Google Scholar]
- Błedzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe Cladocera & Copepoda (Calanoida, Cyclopoida). In Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer International Publishing: New York, NY, USA, 2016; p. 918. [Google Scholar]
- Ruttner-Kolisko, A. Suggestions for biomass calculation of plankton rotifers. Arch. Hydrobiol. 1977, 8, 71–76. [Google Scholar]
- Dumont, H.J.; Van Der Velde, I.; Dumont, S. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 1975, 19, 75–97. [Google Scholar] [CrossRef]
- Culver, D.A.; Boucherle, M.; Bean, D.J.; Fletcher, J.W. Biomass of freshwater crustacean zooplankton from length- weight regressions. Can. J. Fish. Aquat. Sci. 1985, 42, 1380–1390. [Google Scholar] [CrossRef]
- Gyllström, M.; Hansson, L.; Jeppesen, E.; Criado, F.; Gross, E.; Irvine, K.; Kairesalo, T.; Kornijów, R.; Miracle, M.R.; Nykänen, M.; et al. The role of climate in shaping zooplankton communities of shallow lakes. Limnol. Oceanogr. 2005, 50, 2008–2021. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.L.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish Lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kuczyńska-Kippen, N. The distribution of rotifers (Rotifera) within a single Myriophyllum bed. Hydrobiologia 2003, 506, 327–331. [Google Scholar] [CrossRef]
- Brucet, S.; Boix, D.; Quintana, X.D.; Jensen, E.; Nathansen, L.W.; Trochine, C.; Meerhoff, M.; Gascón, S.; Jeppesena, E. Factors influencing zooplankton size structure at contrasting temperatures in coastal shallow lakes: Implications for effects of climate change. Limnol. Oceanogr. 2010, 55, 1697–1711. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Jensen, J.P.; Jensen, C.; Faafeng, B.; Hessen, D.O.; Søndergaard, M.; Lauridsen, T.; Brettum, P.; Christoffersen, K. The Impact of Nutrient State and Lake Depth on Top-down Control in the Pelagic Zone of Lakes: A Study of 466 Lakes from the Temperate Zone to the Arctic. Ecosystems 2003, 6, 313–325. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Sondergaard, M.; Fenger-Gron, M.; Bramm, M.E.; Sandby, K.; Møller, P.H.; Rasmussen, H.U. Impact of fish predation on cladoceran body weight distribution and zooplankton grazing in lakes during winter. Freshw. Biol. 2004, 49, 432–447. [Google Scholar] [CrossRef]
- Moreno-Ostos, E.; Palomino-Torres, R.L.; Escot, C.; Blanco, J.M. Planktonic metabolism in a Mediterranean reservoir during a near-surface cyanobacterial bloom. Limnetica 2016, 35, 117–130. [Google Scholar] [CrossRef]
- Engström, J.; Viherluoto, M.; Viitasalo, M. Effects of toxic and nontoxic cyanobacteria on grazing, zooplanktivory and survival of the mysid shrimp Mysis mixta. J. Exp. Mar. Biol. Ecol. 2001, 257, 269–280. [Google Scholar] [CrossRef]
- Lampert, W. Laboratory studies on zooplankton–cyanobacterial interactions. N. Z. J. Mar. Freshw. Res. 1987, 21, 483–490. [Google Scholar] [CrossRef]
- Nandini, S.; Zamora-Barrios, C.A.; Sarma, S.S.S. A Long-Term Study on the Effect of Cyanobacterial Crude Extracts from Lake Chapultepec (Mexico City) on Selected Zooplankton Species. Environ. Toxicol. Chem. 2020, 39, 2409–2414. [Google Scholar] [CrossRef]
- Langeland, A. Biomanipulation development in Norway. Hydrobiologia 1990, 200, 535–540. [Google Scholar] [CrossRef]
- Ger, K.A.; Hansson, L.-A.; Lürling, M. Understanding cyanobacteria–zooplankton interactions in a more eutrophic world. Freshw. Biol. 2014, 59, 1783–1798. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.X.; Tao, M.; Qin, B.Q.; Qi, M.; Niu, Y.; Zhang, J.; Ma, Z.; Xie, P. Large-scale field evidence on the enhancement of small-sized cladocerans by Microcystis blooms in Lake Taihu, China. J. Plankton Res. 2012, 34, 853–863. [Google Scholar] [CrossRef]
- De Bernardi, R.; Giussani, G. Are blue-green algae a suitable food for zooplankton? An overview. Hydrobiologia 1990, 200, 29–41. [Google Scholar] [CrossRef]
- Galir, B.A.; Ternjej, I.; Špoljar, M. Hydrology driven changes in the rotifer trophic structure and implications for food web interactions. Ecohydrology 2018, 11, e1917. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Vicente, E.; Soria, J.M.; Miracle, M.R. Zooplankton changes at six reservoirs in the Ebro watershed, Spain. Limnetica 2021, 40, 279–294. [Google Scholar] [CrossRef]
- Havens, K.E.; Beaver, J.B. Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia 2011, 668, 49–60. [Google Scholar] [CrossRef]
- Vakkilainen, K.; Kairesalo, T.; Hietala, J.; Balayla, D.M.; Bécares, E.; Van de Bund, W.J.; Van Donk, E.; Fernandez-Alaez, M.; Gyllström, M.; Hansson, L.-A.; et al. Response of zooplankton to nutrient enrichment and fish in shallow lakes: A pan-European mesocosm experiment. Freshw. Biol. 2004, 49, 1619–1632. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C. The Ecology of Phytoplankton (Ecology, Biodiversity and Conservation); Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]






| Large | Small | ||||
|---|---|---|---|---|---|
| Cladocera | Copepoda | Cladocera | Rotifera | ||
| Daphnia | Acanthocyclops | Alona | Anuraeopsis | Gastropus | Ploesoma |
| Diaphanosoma | Cyclops | Alonella | Ascomorpha | Hexarthra | Polyarthra |
| Holopedium | Eucyclops | Bosmina | Asplanchna | Hexarthra | Phompolyx |
| Ilyocryptus | Macrocyclops | Ceriodaphnia | Brachionus | Kellicottia | Proales |
| Leydigia | Thermocyclops | Chydorus | Cephalodella | Keratella | Ptygura |
| Macrothrix | Tropocyclops | Moina | Collotheca | Lecane | Squatinella |
| Sida | Copidodiaptomus | Oxyurella | Colurella | Lepadella | Synchaeta |
| Eudiaptomus | Conochilus | Lophocaris | Testudinella | ||
| Neolovenula | Dicranophorus | Macrochaetus | Trichocerca | ||
| Ergasilus | Encentrum | Monommata | Trichotria | ||
| Neoergasilus | Eosphora | Mytilina | Tripleuchlanis | ||
| Euchlanis | Notholca | ||||
| Harpacticoids | Filinia | Notommata | Bdelloids | ||
| Metric | Coefficients | ||||
|---|---|---|---|---|---|
| TP | CHLA | Temp | DO | SD | |
| Density | |||||
| ZOO | 0.18 *** | 0.33 *** | 0.24 *** | −0.14 * | |
| LZOO | 0.26 *** | 0.29 *** | 0.30 *** | 0.16 ** | |
| SZOO | 0.14 * | 0.24 *** | 0.29 ** | ||
| ZOO:CHLA | −0.37 *** | −0.64 *** | −0.14 * | 0.32 *** | |
| ZOO:PHYTO | −0.33 *** | −0.22 *** | |||
| ROT | 0.18** | ||||
| CLAD | 0.16 ** | 0.39 *** | 0.36 *** | −0.16 ** | |
| COP | 0.25 *** | 0.28 *** | 0.28 *** | −0.12 * | −0.17 ** |
| DAPHN | 0.14 * | 0.19 ** | |||
| CYCLO | 0.27 *** | 0.20 ** | 0.18 ** | −0.20 ** | |
| CALA | 0.25 ** | 0.27 ** | 0.27 ** | −0.24 ** | |
| Biomass | |||||
| ZOO | 0.24 *** | 0.37 *** | 0.32 *** | −0.17 ** | |
| LZOO | 0.24 *** | 0.26 *** | 0.27 *** | 0.16 ** | |
| SZOO | 0.21 ** | 0.36 *** | 0.22 *** | −0.12 * | |
| ZOO:CHLA | −0.37 *** | 0.64 *** | −0.14 * | 0.32 *** | |
| ZOO:PHYTO | −0.23 *** | −0.16 ** | |||
| ROT | 0.13 * | 0.23 *** | |||
| CLAD | 0.13 * | 0.34 *** | 0.32 *** | ||
| COP | 0.19 ** | 0.24 *** | 0.26 *** | −0.13 * | |
| DAPHN | 0.16 * | ||||
| CYCLO | 0.24 ** | 0.17 * | 0.15 * | −0.18 ** | |
| CALA | 0.26 ** | 0.28 ** | 0.27 ** | −0.25 ** | |
| Variable | Coefficients | Regression Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | Chla | DO | T | SD | p | r2 | n | ||
| Density | |||||||||
| ZOO | 0.40 *** | 1.04 * | F2 = 40.41 | 0.0001 | 0.1166 | 292 | |||
| LZOO | 0.40 ** | 2.15 *** | F2 = 20.60 | 0.0001 | 0.122 | 280 | |||
| SZOO | 0.35 ** | 0.57 ns | 0.91 ns | F3 = 7.433 | 0.0001 | 0.06 | 291 | ||
| ZOO:CHLA | −1.11 *** | 0.45 ns | F2 = 178.8 | 0.0001 | 0.5474 | 292 | |||
| ZOO:PHYTO | 0.32 ** | −0.80 *** | −1.51 * | F3 = 16.46 | 0.0001 | 0.13 | 288 | ||
| ROT | 0.35 ** | F1 = 9.88 | 0.0018 | 0.02 | 293 | ||||
| CLAD | −0.25 * | 0.76 *** | 2.82 *** | F3 = 27.23 | 0.0001 | 0.2112 | 291 | ||
| DAPHN | 0.36 ** | F1 = 8.557 | 0.0037 | 0.032 | 227 | ||||
| COP | 0.57 ** | −0.91 ns | 2.40 ** | F3 = 13.62 | 0.0001 | 0.1141 | 291 | ||
| CYCLO | 0.46 ** | 1.16 ns | F2 = 9.605 | 0.0001 | 0.07 | 213 | |||
| CALA | 0.45 ** | −0.8304 ns | 1.74 ns | F3 = 6.783 | 0.0002 | 0.1043 | 146 | ||
| Biomass | |||||||||
| ZOO | 0.47 *** | 1.88 *** | F2 = 31.28 | 0.0001 | 0.1708 | 292 | |||
| LZOO | 0.18 ns | 0.26 ns | 1.97 ** | F3 = 11.43 | 0.0001 | 0.0999 | 279 | ||
| SZOO | 0.61 *** | 0.98 ns | F2 = 24.17 | 0.0001 | 0.1361 | 292 | |||
| ZOO:CHLA | −1.03 *** | 0.92 * | F2 = 108.6 | 0.0001 | 0.4226 | 292 | |||
| ZOO:PHYTO | −0.48 *** | −0.70 ns | 1.27 * | F3 = 10.12 | 0.0001 | 0.08 | 288 | ||
| ROT | 0.42 *** | F1 = 16.62 | 0.0001 | 0.05 | 293 | ||||
| CLAD | −0.30 * | 0.74 *** | −0.63 ns | 2.31 ** | F4 = 15.76 | 0.0001 | 0.1672 | 290 | |
| COP | 0.53 ** | 3.47 ** | F2 = 15.07 | 0.0001 | 0.08 | 292 | |||
| DAPHN | 0.29 * | F1 = 5.67 | 0.0189 | 0.02 | 227 | ||||
| CYCLO | 0.48 ** | F1 = 13.45 | 0.0003 | 0.054 | 214 | ||||
| CALA | 0.46 ** | −0.8304 ns | 1.80 ns | F2 = 6.972 | 0.0002 | 0.1073 | 146 | ||
| Metric | Trophic Status | Ecological Potential | ||||
|---|---|---|---|---|---|---|
| Oligo–Meso | Meso–Eutro | Eutro–Hyper | Oligo–Eutro | Good–Moderate | Moderate–Poor | |
| Density | ||||||
| ZOO | * | ** | *** | |||
| LZOO | ** | *** | *** | ** | ||
| SZOO | * | |||||
| ZOO:CHLA | *** | *** | *** | *** | ||
| ZOO:PHYTO | *** | * | *** | |||
| ROT | ||||||
| CLAD | * | ** | *** | *** | ||
| DAPHN | ** | * | * | |||
| COP | ** | ** | *** | |||
| CYCLO | *** | *** | *** | |||
| CALA | * | ** | ** | |||
| Biomass | ||||||
| ZOO | * | ** | *** | *** | ||
| LZOO | * | *** | *** | |||
| SZOO | * | *** | *** | |||
| ZOO:CHLA | *** | ** | *** | *** | *** | |
| ZOO:PHYTO | ||||||
| ROT | * | |||||
| CLAD | * | * | *** | ** | ||
| DAPHN | * | * | * | |||
| COP | * | *** | *** | |||
| CYCLO | *** | * | ** | |||
| CALA | * | ** | ** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Colmenares, M.E.; Sendra, M.D.; Sòria-Perpinyà, X.; Soria, J.M.; Vicente, E. The Use of Zooplankton Metrics to Determine the Trophic Status and Ecological Potential: An Approach in a Large Mediterranean Watershed. Water 2021, 13, 2382. https://doi.org/10.3390/w13172382
Muñoz-Colmenares ME, Sendra MD, Sòria-Perpinyà X, Soria JM, Vicente E. The Use of Zooplankton Metrics to Determine the Trophic Status and Ecological Potential: An Approach in a Large Mediterranean Watershed. Water. 2021; 13(17):2382. https://doi.org/10.3390/w13172382
Chicago/Turabian StyleMuñoz-Colmenares, Manuel E., María D. Sendra, Xavier Sòria-Perpinyà, Juan Miguel Soria, and Eduardo Vicente. 2021. "The Use of Zooplankton Metrics to Determine the Trophic Status and Ecological Potential: An Approach in a Large Mediterranean Watershed" Water 13, no. 17: 2382. https://doi.org/10.3390/w13172382
APA StyleMuñoz-Colmenares, M. E., Sendra, M. D., Sòria-Perpinyà, X., Soria, J. M., & Vicente, E. (2021). The Use of Zooplankton Metrics to Determine the Trophic Status and Ecological Potential: An Approach in a Large Mediterranean Watershed. Water, 13(17), 2382. https://doi.org/10.3390/w13172382