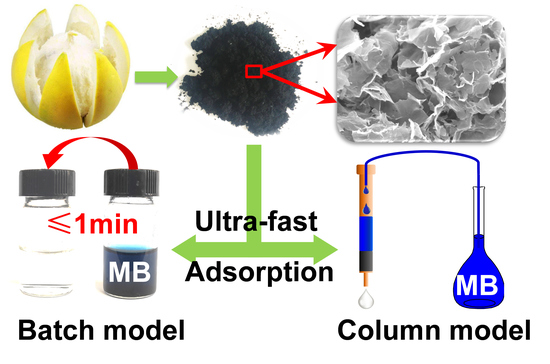
Sheet-like Skeleton Carbon Derived from Shaddock Peels with Hierarchically Porous Structures for Ultra-Fast Removal of Methylene Blue
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of SPACs Adsorbents
2.3. Characterizations of Adsorbents
2.4. Adsorption Experiments
- Batch adsorption. Typically, 10 mg of SPACs adsorbents was placed in a glass vial (20 mL) which containing 10 mL MB solution with a fixed initial concentration of 100 mg/g. Then, the glass vial was sealed and shaken on a SHA-B shaker (Changzhou Tianrui Instrument Co. Ltd., Changzhou, China) with a speed of 200 rpm. After adsorption for a certain time, the solution was filtered by using a syringe-driven filter, which was equipped with a 0.45 μm filter membrane. The concentration of MB in filtrate was determined using a UV-vis spectrophotometer (722N, Shanghai Jinghua Instrument Co. Ltd., Shanghai, China) at a wavelength of 662 nm. The amounts of MB adsorbed per mass of SPACs (q, mg/g) and MB removal rate (R, %) were calculated by means of the following Equations (1) and (2), respectively:where, C0 (mg/g) is the initial concentration of MB, and Ce (mg/g) represents the equilibrium concentration, respectively; V (mL) is the solution volume; m (g) denotes the mass of adsorbent.
- Column adsorption. The column adsorption procedure was performed by using a solid phase extraction device (Shanghai Lichen Instrument Technology Co., Ltd., Shanghai, China) and plastic columns with inner diameter of 5.6 mm and length of 66.3 mm. An AP-02B vacuum pump (Tianjin Automatic Science Instrument Co., Ltd., Tianjin, China) was used to maintain the volumetric flow rate. Briefly, 5 mg of adsorbents were filled to plastic columns with heights of 6 mm, and MB solutions with initial concentration of 10–200 mg/L were passed through the column at a volumetric flow rate of 20 mL/min. The concentrations of solutions collected after through the column were analyzed by using UV-vis spectrophotometer at a wavelength of 662 nm. MB removal rate (R, %) were calculated using abovementioned Equation (2).
3. Results and Discussion
3.1. Characterizations of As-Prepared Adsorbents
3.2. Adsorption Performance of MB with Batch Model
3.2.1. Effect of Contact Time
3.2.2. Effect of Initial Concentration
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Comparisons of Adsorption Capacities with Other Adsorbents
3.6. Reusability of SPAC-8
3.7. Column Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Li, D.; Chai, K.; Yao, X.; Zhou, L.; Wu, K.; Huang, Z.; Yan, J.; Qin, X.; Wei, W.; Ji, H. beta-Cyclodextrin functionalized SBA-15 via amide linkage as a super adsorbent for rapid removal of methyl blue. J. Colloid Interf. Sci. 2021, 583, 100–112. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of Synthetic Dyes—A Review. Water Air Soil Poll. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mo, J.; Du, X.; Zhang, Z.; Zhang, W. Biomimetic dynamic membrane for aquatic dye removal. Water Res. 2019, 151, 243–251. [Google Scholar] [CrossRef]
- Catto, C.; Sanmartin, P.; Gulotta, D.; Troiano, F.; Cappitelli, F. Bioremoval of graffiti using novel commercial strains of bacteria. Sci. Total Environ. 2021, 756, 144075. [Google Scholar] [CrossRef]
- Rachna; Rani, M.; Shanker, U. Synergistic effects of zinc oxide coupled copper hexacyanoferrate nanocomposite: Robust visible-light driven dye degradation. J. Colloid Interf. Sci. 2021, 584, 67–79. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, P.; Wang, Q.; Wu, Y.; Cao, D.; Qiao, Q.A. Construction of Bi2S3-BiOBr nanosheets on TiO2 NTA as the effective photocatalysts: Pollutant removal, photoelectric conversion and hydrogen generation. J. Colloid Interf. Sci. 2021, 585, 459–469. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.M.; Bergmann, C.P.; Fernandes, T.H.M.; Lima, E.C.; Royer, B.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon. J. Hazard. Mater. 2011, 192, 1122–1131. [Google Scholar] [CrossRef]
- Kim, T.S.; Song, H.J.; Dar, M.A.; Lee, H.J.; Kim, D.W. Fast adsorption kinetics of highly dispersed ultrafine nickel/carbon nanoparticles for organic dye removal. Appl. Surf. Sci. 2018, 439, 364–370. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Ullah, A.; Razzaq, H.; Zia, K.M.; Liu, X. Polyvinylidene fluoride nanocomposite super hydrophilic membrane integrated with Polyaniline-Graphene oxide nano fillers for treatment of textile effluents. J. Hazard. Mater. 2021, 403, 123587. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Kiliyankil, V.A.; Takiguchi, S.; Kumashiro, T.; Fugetsu, B.; Sakata, I. Graphene nanosheets homogeneously incorporated in polyurethane sponge for the elimination of water-soluble organic dyes. J. Colloid Interf. Sci. 2021, 584, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Ruotolo, L.A.M.; Cai, W. Functional biobased hydrogels for the removal of aqueous hazardous pollutants: Current status, challenges, and future perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Yu, Y.; Qiao, N.; Wang, D.; Zhu, Q.; Fu, F.; Cao, R.; Wang, R.; Liu, W.; Xu, B. Fluffy honeycomb-like activated carbon from popcorn with high surface area and well-developed porosity for ultra-high efficiency adsorption of organic dyes. Bioresour. Technol. 2019, 285, 121340. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Optimized waste tea activated carbon for adsorption of Methylene Blue and Acid Blue 29 dyes using response surface methodology. Chem. Eng. J. 2011, 175, 233–243. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Fast and efficient adsorptive removal of organic dyes and active pharmaceutical ingredient by microporous carbon: Effect of molecular size and charge. Chem. Eng. J. 2019, 378, 122218. [Google Scholar] [CrossRef]
- Lin, J.; Wang, H.; Ren, E.; Song, Q.; Lan, J. Stomatocyte-like hollow polydopamine nanoparticles for rapid removal of water-soluble dyes from water. Chem. Commun. 2019, 55, 8162–8165. [Google Scholar] [CrossRef] [PubMed]
- Gissawong, N.; Mukdasai, S.; Boonchiangma, S.; Sansuk, S.; Srijaranai, S. A rapid and simple method for the removal of dyes and organophosphorus pesticides from water and soil samples using deep eutectic solvent embedded sponge. Chemosphere 2020, 260, 127590. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Doczekalska, B.; Bartkowiak, M.; Waliszewska, B.; Orszulak, G.; Cerazy-Waliszewska, J.; Pniewski, T. Characterization of chemically activated carbons prepared from miscanthus and switchgrass biomass. Materials 2020, 13, 1654. [Google Scholar] [CrossRef] [Green Version]
- Alcañiz-Monge, J.; Illán-Gómez, M.J. Insight into hydroxides-activated coals: Chemical or physical activation. J. Colloid Interf. Sci. 2008, 318, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillo-Ródenas, M.A.; Juan-Juan, J.; Cazorla-Amorós, D.; Linares-Solano, A. About reactions occurring during chemical activation with hydroxides. Carbon 2004, 42, 1371–1375. [Google Scholar] [CrossRef]
- Lee, D.; Cho, Y.G.; Song, H.K.; Chun, S.J.; Park, S.B.; Choi, D.H.; Lee, S.Y.; Yoo, J.T.; Lee, S.Y. Coffee-driven green activation of cellulose and its use for all-paper flexible supercapacitors. ACS Appl. Mater. Inter. 2017, 9, 22568–22577. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Guo, Q. Progress research on activation mechanism and regeneration of activated carbon. China Powder Sci. Technol. 2008, 5, 48–52. [Google Scholar]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass derived interconnected hierarchical micro-meso-macro- porous carbon with ultrahigh capacitance for supercapacitors. Carbon 2019, 147, 540–549. [Google Scholar] [CrossRef]
- Zhi, L.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsen, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energ. Environ. Sci. 2013, 6, 871–878. [Google Scholar]
- Patino, J.; Lopez-Salas, N.; Gutierrez, M.C.; Carriazo, D.; Ferrer, M.L.; Monte, F.D. Phosphorus-doped carbon-carbon nanotube hierarchical monoliths as true three-dimensional electrodes in supercapacitor cells. J. Mater. Chem. A 2016, 4, 1251–1263. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Sun, B.; Zhou, H.; Li, H. Carbon materials with hierarchical porosity: Effect of template removal strategy and study on their electrochemical properties. Carbon 2018, 130, 680–691. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X.S. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Li, R.; Huang, J.; Li, W.; Li, J.; Cao, L.; Xu, Z.; He, Y.; Yu, A.; Lu, G. Controlling carbon-oxygen double bond and pseudographic structure in shaddock peel derived hard carbon for enhanced sodium storage properties. Electrochim. Acta 2019, 313, 109–115. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Liu, C.; Zhen, Y.; Zhang, W.; Ma, J. A novel 3D adsorbent of reduced graphene oxide-β-cyclodextrin aerogel coupled hardness with softness for efficient removal of bisphenol A. Chem. Eng. J. 2019, 372, 896–904. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Wu, X.; Yuan, X.; Wu, Y. Porous carbon derived from disposable shaddock peel as an excellent catalyst toward VO2+/VO2+ couple for vanadium redox battery. J. Power Sources 2015, 299, 301–308. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl. Mater. Inter. 2013, 5, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lu, R.; Ren, S.; Chen, C.; Chen, Z. Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. [Google Scholar] [CrossRef]
- Dilek, A. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar]
- Geng, J.; Gu, F.; Chang, J. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(VI) and Rhodamine B from wastewater. J. Hazard. Mater. 2019, 375, 174–181. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Ju, H.; Yang, F.; Zhang, L.; Luo, X. Micro-mesoporous divinyl benzene-based polymer for ultrafast, effective and selective removal of cationic dyes. Mater. Chem. Phys. 2020, 255, 123564. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, H.; Zeng, F.; Li, X.; Sun, J.; Li, C.; Lin, H.; Su, Z. HKUST-1 modified ultrastability cellulose/chitosan composite aerogel for highly efficient removal of methylene blue. Carbohyd. Polym. 2021, 255, 117402. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.; Yan, C.; Zhou, S. Green synthesis of loofah fiber-based adsorbents with high adsorption performance for methylene blue. Mater. Lett. 2021, 284, 128929. [Google Scholar] [CrossRef]
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent cellulose–clay Nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Spagnoli, A.A.; Giannakoudakis, D.A.; Bashkova, S. Adsorption of methylene blue on cashew nut shell based carbons activated with zinc chloride: The role of surface and structural parameters. J. Mol. Liq. 2017, 229, 465–471. [Google Scholar] [CrossRef]
- Vunain, E.; Biswick, T. Adsorptive removal of methylene blue from aqueous solution on activated carbon prepared from Malawian baobab fruit shell wastes: Equilibrium, kinetics and thermodynamic studies. Sep. Sci. Technol. 2019, 54, 27–41. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, H.; Pan, Y.; Zhang, M.; Jin, Y. Thermal and pH dual-responsive cellulose microfilament spheres for dye removal in single and binary systems. J. Hazard. Mater. 2019, 377, 88–97. [Google Scholar] [CrossRef]
- Gouthaman, A.; Asir, J.A.; Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M.; Ahamed, M.A.R.; Azarudeen, R.S. Enhanced dye removal using polymeric nanocomposite through incorporation of Ag doped ZnO nanoparticles: Synthesis and characterization. J. Hazard. Mater. 2019, 373, 493–503. [Google Scholar]
- Dhanavel, S.; Nivethaa, E.A.K.; Dhanapal, K.; Gupta, V.K.; Narayanan, V.; Stephen, A. α-MoO3/polyaniline composite for effective scavenging of Rhodamine B, Congo red and textile dye effluent. RSC Adv. 2016, 6, 28871–28886. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Shi, S.L.; Lv, J.P.; Liu, Q.; Nan, F.R.; Jiao, X.Y.; Feng, J.; Xie, S.L. Application of Phragmites australis to remove phenol from aqueous solutions by chemical activation in batch and fixed-bed columns. Environ. Sci. Pollut. Res. 2018, 25, 23917–23928. [Google Scholar] [CrossRef] [PubMed]







| Adsorbents | qe,exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | k1 (/min) | R2 | qe,cal (mg/g) | K2 (g/mg·min) | R2 | ||
| SPAC-0 | 23.3 | 28.1 | 0.1455 | 0.9844 | 24.8 | 0.0077 | 0.9923 |
| SPAC-2 | 100.3 | 78.9 | 0.1747 | 0.9607 | 103.1 | 0.0026 | 0.9946 |
| SPAC-4 | 100.4 | 123.9 | 0.2521 | 0.9598 | 101.0 | 0.0078 | 0.9998 |
| SPAC-6 | 100.4 | 106.6 | 2.482 | 0.8765 | 101.0 | 0.0754 | 0.9999 |
| SPAC-8 | 100.2 | 148.0 | 4.001 | 0.9917 | 100.0 | 0.2000 | 0.9999 |
| Adsorbents | qm,exp (mg/g) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | kL (L/mg) | R2 | nf | Kf | R2 | ||
| SPAC-0 | 128.7 | 127.6 | 0.632 | 0.9999 | 9.15 | 67.5 | 0.8281 |
| SPAC-2 | 278.7 | 274.7 | 0.569 | 0.9972 | 7.63 | 226.6 | 0.7420 |
| SPAC-4 | 339.6 | 337.8 | 0.643 | 0.9997 | 8.43 | 169.6 | 0.7404 |
| SPAC-6 | 362.2 | 359.7 | 0.381 | 0.9994 | 8.31 | 180.5 | 0.7938 |
| SPAC-8 | 432.5 | 434.8 | 0.228 | 0.9993 | 13.6 | 429.9 | 0.8199 |
| Adsorbents | Adsorption Capacity (mg/g) | Adsorption Time (min) | Reference |
|---|---|---|---|
| Magnetic chitosan/graphene oxide | 95.16 | 60 | [41] |
| HKUST-1 modified cellulose/chitosan composite | 526.3 | 900 | [42] |
| Loofah fiber-graft-polyacrylic acid | 302.4 | 3 | [43] |
| Cellulose−clay nanocomposite hydrogels | 782.9 | 20 | [44] |
| Activated carbon derived from cashew nut shells | 476 | 1440 | [45] |
| Activated carbon derived from Malawian baobab fruit shells | 334.45 | 60 | [46] |
| Cellulose microfilament spheres | 497.5 | 180 | [47] |
| SPAC-8 | 432.5 | ≤1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Liu, H.; Xu, S.; Chen, C.; Feng, S.; Long, A. Sheet-like Skeleton Carbon Derived from Shaddock Peels with Hierarchically Porous Structures for Ultra-Fast Removal of Methylene Blue. Water 2021, 13, 2554. https://doi.org/10.3390/w13182554
Dong P, Liu H, Xu S, Chen C, Feng S, Long A. Sheet-like Skeleton Carbon Derived from Shaddock Peels with Hierarchically Porous Structures for Ultra-Fast Removal of Methylene Blue. Water. 2021; 13(18):2554. https://doi.org/10.3390/w13182554
Chicago/Turabian StyleDong, Panlong, Hailin Liu, Shengrui Xu, Changpo Chen, Suling Feng, and Anying Long. 2021. "Sheet-like Skeleton Carbon Derived from Shaddock Peels with Hierarchically Porous Structures for Ultra-Fast Removal of Methylene Blue" Water 13, no. 18: 2554. https://doi.org/10.3390/w13182554
APA StyleDong, P., Liu, H., Xu, S., Chen, C., Feng, S., & Long, A. (2021). Sheet-like Skeleton Carbon Derived from Shaddock Peels with Hierarchically Porous Structures for Ultra-Fast Removal of Methylene Blue. Water, 13(18), 2554. https://doi.org/10.3390/w13182554