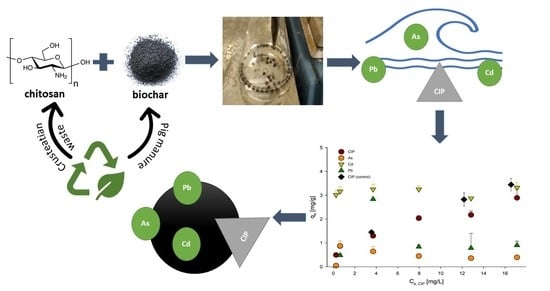
Insights into the Simultaneous Sorption of Ciprofloxacin and Heavy Metals Using Functionalized Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan-Biochar Beads
2.3. CH-BH Characterization
2.4. Adsorption Experiments
2.5. ICP
2.6. Statistical Analysis
3. Results
3.1. Chitosan-Biochar Beads
3.1.1. Fabrication
3.1.2. Characterization
3.2. Adsorption onto CH-BB
3.2.1. Adsorption Potency
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Kinetics
Individual Adsorption Kinetics
Adsorption Kinetics for Pollutant Mixture
Kinetics Modeling
3.2.4. Co-Adsorption of CIP and Heavy Metals
3.2.5. Test with Real Wastewater
4. Discussion
4.1. CH-BB Generation
4.2. Adsoprtion Isotherms
4.3. Adsoprtion in Pollutant Mixture
5. Conclusions
- Arsenic: Physisorption was the main sorption mechanism. In the presence of other heavy metals, the adsorption capacity increased 2-fold due to co-precipitation of As and Pb (mimetite). The potential complexation between CIP and As resulted in decreased sorption of the metalloid.
- Cadmium: Chemisorption was the main sorption mechanism, where intraparticle diffusion was a rate-controlling factor. The addition of other pollutants resulted in a decreased adsorption ability of Cd, suggesting (1) competition over adsorption sites between elements, and (2) CIP–Cd complex formation, which has a higher affinity towards the aqueous phase.
- Lead: Intraparticle diffusion was a rate-controlling factor during the sorption process. Chemisorption was the main sorption mechanism when Pb was tested alone. The addition of other pollutants resulted in sorption disturbance due to (1) co-precipitation of Pb with As, and (2) CIP–Pb complex formation (less free Pb2+ to adsorb onto CH-BB). Moreover, CIP exhibited the greatest influence on the Pb adsorption ability amongst the tested inorganic pollutants.
- Ciprofloxacin: CIP adsorption involved both chemi- and physisorption. The presence of inorganic pollutants did not influence the antibiotic adsorption ability but negatively affected its mobility.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Pobi, K.K.; Satpati, S.; Dutta, S.; Nayek, S.; Saha, R.N.; Gupta, S. Sources Evaluation and Ecological Risk Assessment of Heavy Metals Accumulated within a Natural Stream of Durgapur Industrial Zone, India, by Using Multivariate Analysis and Pollution Indices. Appl. Water Sci. 2019, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Action Is Needed on Chemicals of Major Public Health Concern; Public Health and Environment, World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Soumya, G.; Moogoui, R.; Gupta, D.K. Arsenic: Source, Occurrence, Cycle, and Detection. In Arsenic Contamination in the Environment: The Issues and Solutions; Gupta, D.K., Chatterjee, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–35. [Google Scholar]
- Pop, C.-E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A Effects in Aqueous Environment on Lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Gao, P.; Zhenhong, L.; Huang, S.; Li, K.; Liu, Z.; Xue, G.; Sun, W. Impacts of coexisting antibiotics, antibacterial residues, and heavy metals on the occurrence of erythromycin resistance genes in urban wastewater. Appl. Microbiol. Biotechnol. 2015, 99, 3971–3980. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Conley, Z.C.; Bodine, T.J.; Chou, A.; Zechiedrich, L. Wicked: The untold story of ciprofloxacin. PLOS Pathog. 2018, 14, e1006805. [Google Scholar] [CrossRef] [Green Version]
- Cuprys, A.; Pulicharla, R.; Brar, S.K.; Drogui, P.; Verma, M.; Surampalli, R.Y. Fluoroquinolones metal complexation and its environmental impacts. Co-ord. Chem. Rev. 2018, 376, 46–61. [Google Scholar] [CrossRef]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.C.; Hugie, C.N.; Kile, M.L.; Navab-Daneshmand, T. Association between heavy metals and antibiotic-resistant human pathogens in environmental reservoirs: A review. Front. Environ. Sci. Eng. 2019, 13, 46. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Yue, R.; Sun, X.-F.; Song, C.; Wang, S.-G. Enhanced removal of ciprofloxacin using humic acid modified hydrogel beads. J. Colloid Interface Sci. 2019, 543, 76–83. [Google Scholar] [CrossRef]
- Cuprys, A.; Thomson, P.; Ouarda, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Drogui, P.; Surampalli, R.Y. Ciprofloxacin removal via sequential electro-oxidation and enzymatic oxidation. J. Hazard. Mater. 2019, 389, 121890. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Wan, M.-W.; Kan, C.-C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Zeng, Z.-W.; Tan, X.-F.; Liu, Y.-G.; Tian, S.-R.; Zeng, G.-M.; Jiang, L.-H.; Liu, S.-B.; Li, J.; Liu, N.; Yin, Z.-H. Comprehensive Adsorption Studies of Doxycycline and Ciprofloxacin Antibiotics by Biochars Prepared at Different Temperatures. Front. Chem. 2018, 6, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Gao, B.; Zimmerman, A.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Kim, S.; Igalavithana, A.D.; Hashimoto, Y.; Choi, Y.-E.; Mukhopadhyay, R.; Sarkar, B.; Ok, Y.S. Fe(III) loaded chitosan-biochar composite fibers for the removal of phosphate from water. J. Hazard. Mater. 2021, 415, 125464. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Sun, X.-F.; Liu, J.; Song, C.; Wang, S.-G.; Javed, A. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total. Environ. 2018, 639, 560–569. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 2018, 351, 985–994. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. An Insight into the Adsorption of Diclofenac on Different Biochars: Mechanisms, Surface Chemistry, and Thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuprys, A.; Pulicharla, R.; Lecka, J.; Brar, S.K.; Drogui, P.; Surampalli, R. Ciprofloxacin-metal complexes –stability and toxicity tests in the presence of humic substances. Chemosphere 2018, 202, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Samsuri, W.; Sadegh-Zadeh, F.; Seh-Bardan, B.J. Adsorption of As(III) and As(V) by Fe coated biochars and biochars produced from empty fruit bunch and rice husk. J. Environ. Chem. Eng. 2013, 1, 981–988. [Google Scholar] [CrossRef]
- Long, H.; Zheng, Y.-J.; Peng, Y.-L.; Jin, G.-Z.; Deng, W.-H.; Zhang, S.-C. Comparison of arsenic(V) removal with different lead-containing substances and process optimization in aqueous chloride solution. Hydrometallurgy 2018, 183, 199–206. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem 1959, 63, 1024. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Jiang, B.; Lin, Y.; Mbog, J.C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Zhang, L. Co-adsorption and sequential adsorption of the co-existence four heavy metal ions and three fluoroquinolones on the functionalized ferromagnetic 3D NiFe2O4 porous hollow microsphere. J. Colloid Interface Sci. 2017, 511, 135–144. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Chai, L.; Liu, Y.; Zeng, G.; Wang, X.; Zeng, W.; Shang, M.; Deng, J.; Zhou, Z. Enhancement of As(v) adsorption from aqueous solution by a magnetic chitosan/biochar composite. RSC Adv. 2017, 7, 10891–10900. [Google Scholar] [CrossRef] [Green Version]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.; Wang, H.; Shaheen, S.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lv, J.; Luo, L.; Yang, K.; Lin, Y.; Hu, F.; Zhang, J.; Zhang, S. Arsenate and cadmium co-adsorption and co-precipitation on goethite. J. Hazard. Mater. 2013, 262, 55–63. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Wan, J.; Xue, W.; Lei, L.; Wen, X.; Liu, X.; Chen, S.; Yang, Y.; Li, Z.; et al. Chloro-Phosphate Impregnated Biochar Prepared by Co-Precipitation for the Lead, Cadmium and Copper Synergic Scavenging from Aqueous Solution. Bioresour. Technol. 2019, 293, 122102. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Du, C.; Guo, H.; Wang, Z. Adsorption of Pb(Ii), Cr(Iii), Cu(Ii), Cd(Ii) and Ni(Ii) onto a Vanadium Mine Tailing from Aqueous Solution. J. Hazard. Mater. 2009, 169, 838–846. [Google Scholar] [CrossRef]
- Chen, H.; Ma, L.Q.; Gao, B.; Gu, C. Influence of Cu and Ca cations on ciprofloxacin transport in saturated porous media. J. Hazard. Mater. 2013, 262, 805–811. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, Y.; Gu, X.; Gu, C. Effects of metal cations and fulvic acid on the adsorption of ciprofloxacin onto goethite. Environ. Sci. Pollut. Res. 2014, 22, 609–617. [Google Scholar] [CrossRef]







| Liquid chromatography instrument | Finnigan surveyor LC pump (Thermo Scientific, Mississauga, ON, Canada) |
| Detection | TSQ Quantum access triple quadruple mass spectrometer (Thermo Scientific, Mississauga, ON, Canada) |
| Ionization | Electrospray |
| Column | BetaBasic-18 (100 mm × 2.1 mm × 3 μm) (Thermo Scientific, Mississauga, ON, Canada) |
| Oven temperature | 40 °C |
| Flow rate | 0.3 mL/min |
| SRM mode | Positive |
| Spray voltage | 4000 V |
| Skimmer off-set | 20 V |
| Gas pressure | 1.5 mTorr |
| Capillary temperature | 350 °C |
| Injection volume | 10 μL |
| Internal standard | Ciprofloxacin-d8 (CIP-d8) |
| Mobile phase | A: 0.1% formic acid B: acetonitrile + 0.1% formic acid  |
| Collision energy | CIP: 17 V CIP-d8: 19 V |
| Precursor ions | CIP: 332.3 m/z CIP-d8: 340.3 m/z |
| Product ions | CIP: 245.3 m/z CIP-d8: 249.3 m/z |
| Contaminant (Initial Concentration (mg/L)) | Adsorption Capacity (mg/g) | |
|---|---|---|
| PM-BC | CH-BB | |
Ciprofloxacin (10) | 1.36 ± 0.02 | 1.49 ± 0.06 |
| As (10) | 0.24 ± 0.24 | 1.38 ± 0.15 |
| Cd (10) | 4.88 ± 0.41 | 6.49 ± 0.34 |
| Pb (1) | 0.99 ± 0.004 | 0.95 ± 0.01 |
| Isotherm | Parameter | As | Cd | Pb | CIP |
|---|---|---|---|---|---|
| Langmuir | qm (mg/g) | 9.59 | 451.99 | 2.42 | 15.55 |
| KL (L/mg) | 0.01 | 0.002 | 11.16 | 0.012 | |
| RL | 0.57–1.00 | 0.83–1.00 | 0.04–0.31 | 0.48–0.98 | |
| R2 | 0.96 | 0.99 | 1.00 | 0.91 | |
| E | 0.07 | 3.80 | 0.29 × 10−5 | 0.80 | |
| χ | 0.02 | 0.59 × 10−7 | 1.00 | 0.94 | |
| Freundlich | n | 0.71 | 0.94 | 0.50 | 0.72 |
| KF (L/mg) | 0.26 | 1.05 | 3.51 | 0.31 | |
| R2 | 0.97 | 0.99 | 0.97 | 0.89 | |
| E | 0.04 | 3.44 | 0.12 × 10−3 | 0.97 | |
| χ | 0.80 | 0.26 × 10−5 | 1.00 | 0.89 | |
| Redlich–Peterson | KRP (L/g) | 1.06 × 104 | 7.86 | 26.76 | 12.16 |
| g | 0.29 | 0.07 | 1.00 | 0.28 | |
| aRT ((mg/L)−g) | 4.06 × 104 | 6.46 | 11.15 | 38.99 | |
| R2 | 0.97 | 0.99 | 1.00 | 0.89 | |
| E | 0.04 | 3.45 | 0.29 × 10−5 | 0.97 | |
| χ | 0.81 | 0.26 × 10−5 | 1.00 | 0.89 |
| Pollutant(s) | qe,exp | Pseudo-First Order | Pseudo-Second Order | Elovich | Intraparticle Diffusion | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,m | R2 | E | χ | k2 | qe,m | R2 | E | χ | α | β | R2 | E | χ | kp | I | R2 | E | χ | |||
| As | Individually | 0.15 ± 0.02 | 1.10 | 0.15 | 0.81 | 0.68 × 10−5 | 0.99 | 8.47 | 0.17 | 0.70 | 0.11 × 10−4 | 0.99 | 0.64 | 36.50 | 0.51 | 0.18 × 10−4 | 0.99 | 0.020 | 0.069 | 0.26 | 0.26 × 10−4 | 0.99 |
| Metal mixture | 0.27 ± 0.02 | 0.96 | 0.27 | 0.96 | 0.25 × 10−5 | 0.99 | 5.41 | 0.30 | 0.93 | 0.41 × 10−3 | 1.00 | 2.01 | 22.18 | 0.77 | 0.14 × 10−2 | 0.99 | 0.035 | 0.14 | 0.50 | 0.31 × 10−2 | 0.99 | |
| Metal mixture + CIP | 0.14 ± 0.02 | 1.03 | 0.15 | 0.76 | 0.87 × 10−5 | 0.99 | 6.84 | 0.17 | 0.71 | 0.11 × 10−2 | 0.99 | 0.42 | 32.21 | 0.57 | 0.17 × 10−2 | 0.99 | 0.025 | 0.057 | 0.35 | 0.26 × 10−2 | 0.99 | |
| Cd | Individually | 0.99 ± 0.004 | 1.00 | 0.87 | 0.93 | 0.45 × 10−4 | 0.99 | 1.28 | 0.98 | 0.99 | 0.76 × 10−5 | 1.00 | 3.49 | 5.98 | 0.97 | 0.18 × 10−4 | 0.99 | 0.15 | 0.35 | 0.80 | 0.12 × 10−3 | 0.99 |
| Metal mixture | 0.75 ± 0.04 | 0.27 | 0.67 | 0.90 | 0.37 × 10−2 | 0.99 | 0.49 | 0.75 | 0.95 | 0.18 × 10−2 | 0.99 | 0.54 | 6.37 | 0.99 | 0.44 × 10−3 | 0.99 | 0.13 | 0.10 | 0.96 | 0.14 × 10−2 | 0.99 | |
| Metal mixture + CIP | 0.66 ± 0.01 | 0.58 | 0.58 | 0.92 | 0.27 × 10−2 | 0.99 | 1.08 | 0.66 | 0.97 | 0.11 × 10−2 | 1.00 | 0.95 | 7.69 | 0.96 | 0.14 × 10−2 | 0.99 | 0.12 | 0.15 | 0.82 | 0.59 × 10−2 | 0.99 | |
| Pb | Individually | 0.68 ± 0.08 | 0.30 | 0.66 | 0.88 | 0.50 × 10−4 | 0.99 | 0.59 | 0.71 | 0.93 | 0.30 × 10−4 | 1.00 | 0.79 | 7.91 | 0.99 | 0.67 × 10−5 | 0.99 | 0.09 | 0.16 | 0.94 | 0.28 × 10−4 | 0.99 |
| Metal mixture | 0.83 ± 0.01 | 2.50 | 0.67 | 0.53 | 0.01 | 0.99 | 3.36 | 0.74 | 0.74 | 0.89 × 10−2 | 0.99 | 16.47 | 10.79 | 0.95 | 0.14 × 10−2 | 0.99 | 0.072 | 0.40 | 0.92 | 0.27 × 10−2 | 0.99 | |
| Metal mixture + CIP | 0.61 ± 0.01 | 6.37 | 0.48 | 0.33 | 0.76 × 10−2 | 0.99 | 20.39 | 0.50 | 0.41 | 0.55 × 10−2 | 0.99 | 566.01 | 23.16 | 0.71 | 0.35 × 10−2 | 0.99 | 0.039 | 0.36 | 0.85 | 0.20 × 10−2 | 0.99 | |
| CIP | Individually | 0.37 ± 0.03 | 10.07 | 0.38 | 0.71 | 0.10 × 10−4 | 0.99 | 175 | 0.39 | 0.70 | 0.11 × 10−4 | 1.00 | 1.25 × 1019 | 131.07 | 0.68 | 0.12 × 10−4 | 0.99 | 0.006 | 0.37 | 0.7 | 0.11 × 10−4 | 0.99 |
| Metal mixture + CIP | 0.39 ± 0.01 | 0.77 | 0.39 | 0.74 | 0.26 × 10−2 | 0.99 | 4.51 | 0.41 | 0.84 | 0.16 × 10−2 | 0.99 | 4.40 | 17.47 | 0.78 | 0.22 × 10−2 | 0.99 | 0.048 | 0.20 | 0.59 | 0.42 × 10−2 | 0.99 | |
| Adsorbent | As (% Removed) | Cd (% Removed) | Pb (% Removed) | CIP (% Removed) |
|---|---|---|---|---|
| CH-BB | 4.50 ± 0.76 | 92.86 ± 0.68 | 99.99 ± 0.03 | 34.95 ± 4.03 |
| PM-BC | NR | 42.54 ± 6.25 | 99.74 ± 0.07 | 43.57 ± 6.71 |
| GAC | NR | 31.20 ± 0.00 | 99.52 ± 0.12 | 87.93 ± 6.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuprys, A.; Maletskyi, Z.; Rouissi, T.; Ratnaweera, H.; Brar, S.K.; Knystautas, E.; Drogui, P. Insights into the Simultaneous Sorption of Ciprofloxacin and Heavy Metals Using Functionalized Biochar. Water 2021, 13, 2768. https://doi.org/10.3390/w13192768
Cuprys A, Maletskyi Z, Rouissi T, Ratnaweera H, Brar SK, Knystautas E, Drogui P. Insights into the Simultaneous Sorption of Ciprofloxacin and Heavy Metals Using Functionalized Biochar. Water. 2021; 13(19):2768. https://doi.org/10.3390/w13192768
Chicago/Turabian StyleCuprys, Agnieszka, Zakhar Maletskyi, Tarek Rouissi, Harsha Ratnaweera, Satinder Kaur Brar, Emile Knystautas, and Patrick Drogui. 2021. "Insights into the Simultaneous Sorption of Ciprofloxacin and Heavy Metals Using Functionalized Biochar" Water 13, no. 19: 2768. https://doi.org/10.3390/w13192768
APA StyleCuprys, A., Maletskyi, Z., Rouissi, T., Ratnaweera, H., Brar, S. K., Knystautas, E., & Drogui, P. (2021). Insights into the Simultaneous Sorption of Ciprofloxacin and Heavy Metals Using Functionalized Biochar. Water, 13(19), 2768. https://doi.org/10.3390/w13192768