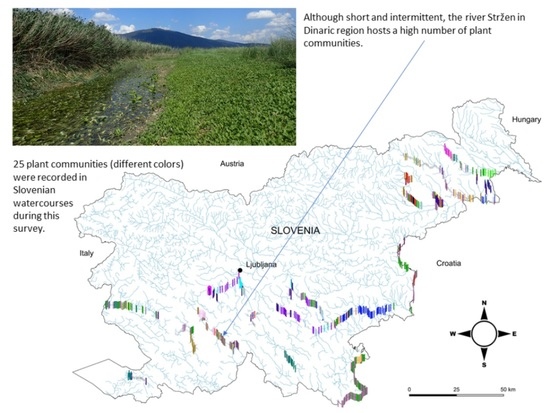
Distribution of Vascular Plant Communities in Slovenian Watercourses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Macrophyte Data Set
2.3. Environment Assessment
2.4. Data Analyses and Classification of Aquatic Plant Communities
2.5. The Influence of Environmental Factors on Macrophyte Community Composition
3. Results
3.1. Macrophyte Taxa and Their Growth Forms
3.2. The Relationships between Growth Forms and Environmental Factors
3.3. Relationships between Species and Environmental Factors
3.4. Macrophyte Communities
4. Discussion
4.1. Species Presence and Abundance
4.2. The Relationships between Macrophyte Presence and Abundance, and Environmental Factors
4.3. Plant Communities
- Class Lemnetea O. de Bolòs et Masclans 1955
- 1.1.
- Alliance Stratiotion Den Hartog et Segal 1964
- 1 Association Ceratophylletum demersi Corillion 1957
- Class Potamogetonetea Klika in Klika et Novák 1941
- 2.1.
- Alliance Potamogetonion Libbert 1931
- 2 Association Potametum denso-nodosi de Bolós 1957
- 3 Association Myriophylletum verticillati Gaudet ex Šumberová in Chytrý 2011
- 4 Association Potamogetono pectinati-Myriophylletum spicati Rivas Goday 1964
- 5 Community with Myriophyllum spicatum
- 6 Association Potametum pectinati Carstensen ex Hilbig 1971
- 7 Association Potametum crispi Soó 1927
- 8 Association Potametum perfoliati Miljan 1933
- 9 Association Potametum natantis Hild 1959
- 10 Association Elodeetum canadensis Nedelcu 1967
- 2.2.
- Alliance Nymphaeion albae Oberdorfer 1957
- 11 Association Nymphaeo albae-Nupharetum luteae Nowiński 1927
- 2.3.
- Ranunculion aquatilis Passarge ex Theurillat in Theurillat et al. 2015
- 12 Association Potamo crispi-Ranunculetum trichophylli Imchenetzky 1926
- Class Bidentetea tripartitae Tüxen et al. ex von Rochow 1951
- 3.1.
- Alliance Bidention tripartitae Nordhagen ex Klika et Hadač 1944
- 13 Association Polygonetum hydropiperis Passarge 1965
- Class Phragmito-Magnocaricetea Klika in Klika et Novák 1941
- 4.1.
- Alliance Phragmition communis Koch 1926
- 14 Association Phragmitetum australis Savič 1926
- 15 Association Glycerio-Sparganietum neglecti Koch 1926
- 16 Association Schoenoplectetum lacustris Chouard 1924
- 17 Association Phalaridetum arundinaceae Libbert 1931
- 4.2.
- Alliance Eleocharito palustris-Sagittarion sagittifoliae Passarge 1964
- 18 Association Oenantho aquaticae-Rorippetum amphibiae Lohmeyer 1950
- 19 Association Alopecuro-Alismatetum plantaginis-aquaticae Bolbrinker 1984
- 20 Association Sagittario sagittifoliae-Sparganietum emersi Tüxen 1953
- 21 Association Eleocharito palustris-Hippuridetum vulgaris Passarge 1964
- 4.3.
- Alliance Glycerio-Sparganion Br.-Bl. et Sissingh in Boer 1942
- 22 Association Glycerio notatae-Veronicetum beccabungae Landucci et al. 2020
- 23 Association Beruletum erectae Roll 1938
- 24 Association Mentho aquaticae-Oenanthetum fistulosae ass. nova
- Class Molinio-Arrhenatheretea Tx. 1937
- 5.1.
- Alliance Potentillion anserinae Tx. 1947
- 25 Association Rumici crispi-Agrostietum stoloniferae Moor 1958
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cluster Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| number of stretches | 6 | 31 | 10 | 10 | 5 | 31 | 6 | 8 | 7 | 12 | 26 | 6 | 109 | 4 | 4 | 5 | 226 | 30 | 5 | 27 | 32 | 23 | 18 | 87 | 18 | 13 | 15 | 7 | 38 | 19 | 11 | 18 | 4 | 35 |
| dominant species of the plant associations | ||||||||||||||||||||||||||||||||||
| Ceratophyllum demersum | 63 | 37 | 36 | . | . | . | . | 5 | . | . | . | . | . | . | . | 3 | 0 | . | . | 1 | . | . | 2 | 0 | . | . | . | . | 1 | . | . | . | . | . |
| Potamogeton nodosus | 22 | 31 | 63 | 66 | . | 16 | 39 | 13 | 6 | . | 0 | . | 4 | . | . | 1 | 1 | 4 | . | 4 | . | 9 | 15 | 18 | . | . | 6 | . | . | 1 | . | . | . | 3 |
| Myriophyllum verticillatum | 3 | . | . | 2 | 63 | . | . | 2 | . | . | . | . | 1 | . | . | . | 0 | . | . | 3 | 0 | . | . | 0 | . | 3 | . | . | 1 | . | . | 0 | . | 2 |
| Myriophyllum spicatum | 26 | 27 | 22 | 23 | 39 | 64 | 63 | 21 | 42 | 9 | 3 | . | 15 | . | . | . | 1 | 11 | . | 13 | 4 | 22 | 19 | 33 | 5 | 3 | 4 | . | 3 | 2 | . | 12 | 13 | 7 |
| Potamogeton pectinatus | 18 | 24 | 29 | . | 15 | 2 | 59 | 63 | 39 | . | 0 | . | 0 | . | . | 14 | 0 | . | . | 0 | 1 | 6 | 6 | 1 | . | . | . | . | 1 | 0 | . | 8 | . | 1 |
| Polygonum hydropiper/mite | 1 | . | . | 1 | 2 | 2 | 5 | 6 | 45 | 6 | 2 | 1 | 0 | . | . | 26 | 0 | 1 | . | 2 | . | . | 8 | 0 | . | 2 | 2 | . | 0 | 1 | 1 | . | . | 1 |
| Phragmites australis | 9 | 1 | 1 | 8 | 13 | 2 | . | 4 | . | 63 | 3 | 3 | 1 | 8 | 13 | 1 | 1 | 22 | . | 1 | 0 | 2 | 0 | 1 | . | 3 | 3 | 1 | 4 | 1 | 5 | . | . | 2 |
| Phalaris arundinacea | 7 | 2 | 0 | 20 | 33 | 7 | 28 | 12 | 42 | 2 | 38 | 7 | 5 | 2 | . | 9 | 4 | 4 | 19 | 11 | 6 | 9 | 13 | 2 | 5 | 69 | 5 | . | 9 | 9 | 15 | 2 | . | 7 |
| Agrostis stolonifera agg. | . | . | . | . | . | . | 22 | . | 13 | . | 5 | 42 | 1 | 4 | 8 | . | 2 | 2 | 3 | 2 | . | 2 | 3 | 0 | . | 2 | 2 | . | 2 | 4 | 1 | . | . | 1 |
| Rorippa amphibia | . | . | . | . | . | 0 | 8 | . | 0 | . | 3 | . | 0 | 44 | . | . | 1 | 4 | 9 | . | 5 | 0 | 0 | . | 2 | 4 | 6 | . | . | 0 | . | 0 | . | . |
| Veronica beccabunga | 1 | . | . | 2 | 4 | . | . | 1 | . | . | 2 | . | 0 | . | 38 | . | 1 | . | . | . | 0 | . | 4 | 0 | . | 2 | . | . | 1 | . | . | . | . | 1 |
| Alisma plantago-aquatica | 1 | . | . | 1 | . | 2 | 8 | 1 | 5 | 5 | 1 | 2 | 0 | 10 | . | 29 | 1 | 2 | . | 3 | . | 0 | 6 | 1 | . | 3 | 2 | . | 1 | 2 | 0 | . | . | 2 |
| Oenanthe fistulosa | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 0 | . | 63 | . | 4 | . | . | . | 3 | 5 | . | . | . | . | . | . | . | . |
| Potamogeton crispus | 3 | 0 | 0 | 7 | . | 2 | 19 | 0 | 18 | 6 | 1 | . | 3 | . | . | 1 | 1 | 2 | . | 36 | 13 | 13 | 9 | 2 | . | 0 | 0 | 0 | 3 | 5 | 0 | 3 | 8 | 9 |
| Ranunculus trichophyllus | 3 | 7 | 12 | . | . | . | . | 2 | . | . | . | 3 | 1 | . | . | . | 1 | 1 | . | 2 | 27 | 3 | 2 | 2 | 1 | 0 | 5 | . | 2 | 7 | . | 1 | 5 | 2 |
| Potamogeton perfoliatus | 5 | 8 | 2 | . | . | 6 | 4 | 10 | 8 | 1 | 1 | . | 2 | . | . | . | 1 | 1 | . | 0 | 7 | 43 | . | 3 | 15 | . | 0 | 31 | 0 | 5 | . | 13 | 1 | 9 |
| Sparganium erectum agg. | 6 | 0 | 3 | 8 | 5 | 2 | 13 | 10 | . | 11 | 3 | 11 | 1 | . | . | 3 | 1 | 5 | . | 4 | . | 1 | 41 | 2 | . | . | 2 | . | 3 | 10 | 15 | . | . | 1 |
| Schoenoplectus lacustris | 3 | 11 | 5 | 4 | . | 1 | . | 7 | . | . | 3 | . | 1 | 2 | . | . | 1 | 2 | 4 | . | 12 | 2 | 1 | 1 | 57 | . | 5 | . | 1 | 1 | . | 15 | . | 1 |
| Nuphar luteum | . | . | . | 12 | . | 0 | 23 | 5 | . | 3 | . | . | 0 | . | . | . | 1 | 17 | . | . | 0 | 1 | . | 0 | 4 | . | 65 | . | . | 7 | . | 19 | . | 6 |
| Callitriche spp. | 1 | . | . | 2 | 2 | 1 | 1 | 7 | 32 | 0 | 0 | . | 0 | . | . | 6 | 1 | . | 1 | 4 | 4 | 0 | 5 | . | . | 10 | . | 38 | 15 | 19 | 4 | 6 | 27 | 5 |
| Berula erecta | 1 | 0 | 0 | 3 | 17 | 0 | 1 | 11 | . | 8 | 3 | 1 | 1 | . | . | . | 1 | 3 | . | 4 | . | 1 | 4 | 0 | . | 5 | 4 | 38 | 24 | 13 | 65 | 7 | 27 | 3 |
| Sparganium emersum | 8 | 6 | . | 8 | 8 | 4 | 5 | 11 | 16 | . | . | . | 0 | . | . | . | 1 | 3 | . | 1 | . | 1 | 1 | 0 | . | 3 | 7 | 29 | 1 | 47 | . | 18 | 1 | 10 |
| Butomus umbellatus | . | . | . | . | . | . | 46 | . | 13 | . | 1 | . | 0 | . | . | . | 1 | 0 | . | . | 0 | 2 | . | . | . | . | 0 | . | . | . | . | . | . | . |
| Potamogeton natans | 9 | 1 | . | . | 8 | 1 | . | 21 | . | 11 | . | . | 0 | . | . | . | 0 | 7 | . | 2 | 3 | 9 | . | 1 | 1 | 1 | . | 0 | . | 15 | 3 | 51 | 10 | 13 |
| Hippuris vulgaris | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 | 1 | . | . | 7 | . | . | . | 1 | . | 4 | 0 | 1 | . | . | 7 | 63 | 2 |
| Elodea canadensis | 1 | 0 | 2 | 0 | 8 | 3 | . | 9 | . | . | . | . | 0 | . | . | . | 1 | . | . | 10 | . | 2 | 2 | 0 | . | . | . | . | 2 | 5 | 3 | 15 | 38 | 49 |
| subdominant taxa | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| Najas marina | . | 14 | 34 | . | . | 1 | 5 | 0 | . | . | . | . | 0 | . | . | . | 0 | . | . | 0 | . | . | 1 | 0 | . | . | . | . | . | . | . | . | . | . |
| Zannichellia palustris | . | . | . | 10 | 8 | . | . | . | . | . | . | . | 0 | . | . | . | 0 | . | . | . | . | 0 | . | 0 | . | 5 | . | . | 2 | . | 0 | . | . | . |
| Potamogeton berchtoldii | . | . | . | . | 26 | 2 | . | 2 | . | . | 1 | . | 1 | . | . | 3 | 0 | . | . | 1 | . | . | 11 | 0 | . | 2 | . | . | 3 | . | 1 | . | . | 1 |
| Iris pseudacorus | 9 | 0 | 1 | . | 13 | 1 | 28 | 9 | 8 | 3 | 6 | 1 | 1 | . | 8 | 2 | 1 | 2 | . | 4 | 0 | 1 | 4 | 1 | 1 | 2 | 1 | 3 | 5 | 3 | 4 | 1 | . | 4 |
| Lythrum salicaria | . | . | . | 2 | 8 | 1 | 5 | 1 | 5 | 7 | 10 | . | 1 | 1 | . | 2 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 12 | 2 | 1 | . | 3 | 1 | 2 | 0 | . | 2 |
| Leersia oryzoides | . | . | . | 2 | . | 1 | . | 6 | . | . | 5 | . | 1 | . | . | . | 0 | . | . | . | . | . | 8 | 0 | . | . | . | . | . | . | . | . | . | . |
| Ranunculus circinatus | 1 | 1 | 11 | . | . | 1 | . | 5 | . | . | . | 9 | 0 | . | . | . | 0 | . | 5 | 2 | 0 | . | . | 0 | 1 | 1 | . | . | 0 | 2 | . | 1 | . | 4 |
| Sium latifolium | . | . | . | . | . | . | . | . | . | . | . | . | . | 5 | . | . | 0 | 1 | 1 | . | 2 | 0 | . | . | 1 | . | 2 | . | . | . | . | . | . | . |
| Nasturtium officinale | 6 | 1 | . | . | 36 | 1 | . | 9 | . | . | 0 | . | 0 | . | 8 | . | 1 | 0 | . | 7 | 1 | 0 | 1 | 0 | . | 11 | . | . | 16 | 0 | 3 | 5 | 5 | 5 |
| Juncus effusus | . | . | . | . | . | . | . | . | . | 0 | 3 | . | 0 | . | 23 | 3 | 0 | 0 | . | 2 | . | . | 2 | 0 | . | . | . | . | . | . | 0 | . | . | . |
| Sparganium spp. | . | . | . | 9 | . | 3 | . | . | . | . | 1 | 3 | 1 | . | 8 | . | 1 | 3 | 1 | 4 | 21 | 4 | . | 2 | 16 | . | 3 | . | 1 | . | . | . | . | 2 |
| Mentha aquatica | 3 | . | . | 3 | 20 | 0 | . | . | . | 5 | 2 | 3 | 0 | 4 | . | 1 | 2 | 3 | 29 | 1 | 13 | 1 | 3 | 0 | 6 | 2 | 7 | . | 7 | 0 | 14 | 0 | 4 | 1 |
| Myosotis scorpioides agg. | 10 | . | . | 2 | 10 | 0 | . | 9 | . | 5 | 1 | 3 | 0 | 2 | . | . | 3 | 1 | 14 | 5 | 11 | 2 | 2 | 0 | 2 | 2 | 5 | 18 | 4 | 0 | 2 | 8 | 2 | 3 |
| Alisma spp. | . | . | . | . | . | 0 | . | . | . | . | 1 | 3 | 0 | 2 | . | . | 1 | 0 | 15 | . | 4 | 1 | . | 0 | 5 | 2 | 0 | . | . | . | . | . | . | . |
| Polygonum amphibium | . | . | . | . | . | . | . | . | . | . | 4 | 1 | . | 2 | . | . | 1 | 1 | 7 | . | 2 | 0 | . | . | 3 | 14 | 6 | . | . | . | . | . | . | . |
| Potamogeton lucens | . | . | . | . | . | . | . | 2 | . | . | . | . | 2 | . | . | . | 0 | 5 | 4 | . | 1 | 4 | . | 1 | 6 | . | 9 | 10 | 1 | 13 | . | 23 | 1 | 4 |
| Veronica anagallis-aquatica | 3 | 9 | 3 | 0 | . | 1 | . | 7 | . | 3 | 0 | . | 0 | . | . | . | 1 | 1 | 4 | 9 | 11 | 2 | 3 | 0 | . | 8 | 0 | 6 | 12 | 0 | 13 | 13 | 23 | 6 |
| Sagittaria sagittifolia | . | . | . | . | . | . | . | 5 | . | . | . | . | . | . | . | . | 0 | 1 | . | . | 0 | 4 | . | 1 | . | . | 0 | . | . | 0 | . | 19 | . | 0 |
| Avg. nr. of species | 12.5 | 6.8 | 19.6 | 5.3 | 13.7 | 12.5 | 12.9 | 8.2 | 8.3 | 7.3 | 4.5 | 7.8 | 4.3 | . | 6.5 | 8.8 | 14.0 | 7.8 | 14.6 | 10.0 | 11.8 | 4.2 | 13.6 | 9.3 | 11.5 | 6.7 | 8.2 | 8.8 | 9.6 | 11.5 | . | 10.7 | ||
References
- Ward, J.V. The Four-Dimensional Nature of Lotic Ecosystems. J. N. Am. Benthol. Soc. 1989, 8, 2–8. [Google Scholar] [CrossRef]
- Settele, J.; Scholes, R.; Betts, R.A.; Bunn, S.; Leadley, P.; Nepstad, D.; Overpeck, J.T.; Angel Taboada, M.; Adrian, R.; Allen, C.; et al. 2014: Terrestrial and inland water systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability: Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1–32. [Google Scholar]
- Stanford, J.A. Rivers in the landscape: Introduction to the special issue on riparian and groundwater ecology. Freshw. Biol. 1998, 40, 402–406. [Google Scholar] [CrossRef]
- Petts, G.E. Rivers: Dynamic components of catchment ecosystems. In The River’s Handbook: Hydrological and Ecological Principles; Calow, P., Petts, G.E., Eds.; Blackwell Science: Oxford, UK, 1994; pp. 3–22. [Google Scholar]
- Boulton, A.J.; Brock, M.A. Australian Freshwater Ecology: Processes and Management; Gleneagles Publishing: Adelaide, Australia, 1999; p. 118. [Google Scholar]
- Baattrup-Pedersen, A.; Larsen, S.E.; Riis, T. Long-term effects of stream management on plant communities in two Danish lowland streams. Hydrobiology 2002, 481, 33–45. [Google Scholar] [CrossRef]
- Kuhar, U.; Gregorc, T.; Renčelj, M.; Šraj-Kržič, N.; Gaberščik, A. Distribution of macrophytes and condition of the physical environment of streams flowing through agricultural landscape in north-eastern Slovenia. Limnologica 2007, 37, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Kuhar, U.; Germ, M.; Gaberščik, A. Macrophytes of watercourses in the Slovenian Danube Basin. In Macrophytes of the River Danube Basin; Janauer, G.A., Gaberščik, A., Květ, J., Germ, M., Exler, N., Eds.; Academia: Praha, Czech Republic, 2018; pp. 234–251. [Google Scholar]
- Petersen, R.C. The RCE: A Riparian, Channel, and Environmental Inventory for small streams in the agricultural landscape. Freshw. Biol. 1992, 27, 295–306. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Ciecierska, H.; Kolada, A.; Schneider, S.C.; Szwabinska, M.; Ruszczynska, J. Parameters structuring macrophyte communities in rivers and lakes–results from a case study in North-Central Poland. Knowl. Manag. Aquat. Ecosyst. 2014, 415, 8. [Google Scholar] [CrossRef] [Green Version]
- Kuhar, U.; Germ, M.; Gaberščik, A.; Urbanič, G. Development of a River Macrophyte Index (RMI) for assessing river ecological status. Limnologica 2011, 41, 235–243. [Google Scholar] [CrossRef]
- Haury, J. Patterns of macrophyte distribution within a Breton brook compared with other study scales. Landsc. Urban Plan. 1995, 31, 349–361. [Google Scholar] [CrossRef]
- Haslam, S. The evaluation of river pollution using vegetation in the Maltese islands. Fresenius Environ. Bull. 2000, 9, 347–351. [Google Scholar]
- Verschoren, V.; Schoelynck, J.; Cox, T.; Schoutens, K.; Temmerman, S.; Meire, P. Opposing effects of aquatic vegetation on hydraulic functioning and transport of dissolved and organic particulate matter in a lowland river: A field experiment. Ecol. Eng. 2017, 105, 221–230. [Google Scholar] [CrossRef]
- Bakker, E.; Van Donk, E.; Declerck, S.; Helmsing, N.; Hidding, B.; Nolet, B. Effect of macrophyte community composition and nutrient enrichment on plant biomass and algal blooms. Basic Appl. Ecol. 2010, 11, 432–439. [Google Scholar] [CrossRef]
- Vestergaard, O.; Sand-Jensen, K. Alkalinity and trophic state regulate aquatic plant distribution in Danish lakes. Aquat. Bot. 2000, 67, 85–107. [Google Scholar] [CrossRef]
- Haslam, S.M. River Plants: The Macrophytic Vegetation of Watercourses; Cardigan, Forrest Text: Ceredigion, UK, 2006; p. 450. [Google Scholar]
- Franklin, P.; Dunbar, M.; Whitehead, P. Flow controls on lowland river macrophytes: A review. Sci. Total. Environ. 2008, 400, 369–378. [Google Scholar] [CrossRef]
- Chambers, P.A.; Prepas, E.E.; Hamilton, H.R.; Bothwell, M.L. Current Velocity and Its Effect on Aquatic Macrophytes in Flowing Waters. Ecol. Appl. 1991, 1, 249–257. [Google Scholar] [CrossRef]
- Dodds, W.K.; Biggs, B.J.F. Water Velocity Attenuation by Stream Periphyton and Macrophytes in Relation to Growth Form and Architecture. J. North Am. Benthol. Soc. 2002, 21, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Biggs, B.J.F. Hydraulic habitat of plants in streams. Regul. Rivers Res. Manag. 1996, 12, 131–144. [Google Scholar] [CrossRef]
- Riis, T.; Biggs, B.J.F. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol. Oceanogr. 2003, 48, 1488–1497. [Google Scholar] [CrossRef] [Green Version]
- Riis, T.; Suren, A.M.; Clausen, B.; Sand-Jensen, K. Vegetation and flow regime in lowland streams. Freshw. Biol. 2008, 53, 1531–1543. [Google Scholar] [CrossRef]
- Fox, A.M. Macrophytes. In The River’s Handbook: Hydrological and Ecological Principles; Calow, P., Petts, G.E., Eds.; Blackwell Science: Oxford, UK, 1992; pp. 216–233. [Google Scholar]
- Murphy, K.J.; Rørslett, B.; Springuel, I. Strategy analysis of submerged lake macrophyte communities: An international example. Aquat. Bot. 1990, 36, 303–323. [Google Scholar] [CrossRef]
- Riis, T.; Tank, J.L.; Reisinger, A.J.; Aubenau, A.; Roche, K.R.; Levi, P.S.; Baattrup-Pedersen, A.; Alnoee, A.B.; Bolster, D. Riverine macrophytes control seasonal nutrient uptake via both physical and biological pathways. Freshw. Biol. 2019, 65, 178–192. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Larsen, S.E.; Riis, T. Composition and richness of macrophyte communities in small Danish streams-influence of environmental factors and weed cutting. Hydrobiologia 2003, 495, 171–179. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Riis, T. Macrophyte diversity and composition in relation to substratum characteristics in regulated and unregulated Danish streams. Freshw. Biol. 1999, 42, 375–385. [Google Scholar] [CrossRef]
- Chambers, P.A.; Lacoul, P.; Murphy, K.J.; Thomaz, S.M. Global diversity of aquatic macrophytes in freshwater. In Freshwater Animal Diversity Assessment; Springer: Berlin, Germany, 2008; pp. 9–26. [Google Scholar]
- Pip, E. Water temperature and freshwater macrophyte distribution. Aquat. Bot. 1989, 34, 367–373. [Google Scholar] [CrossRef]
- Léonard, R.; Legendre, P.; Jean, M.; Bouchard, A. Using the landscape morphometric context to resolve spatial patterns of submerged macrophyte communities in a fluvial lake. Landsc. Ecol. 2007, 23, 91–105. [Google Scholar] [CrossRef]
- Gacia, E.; Ballesteros, E.; Camarero, L.; Delgado, O.; Palau, A.; Riera, J.L.; Catalan, J. Macrophytes from lakes in the eastern Pyrenees: Community composition and ordination in relation to environmental factors. Freshw. Biol. 1994, 32, 73–81. [Google Scholar] [CrossRef]
- Manzo, L.; Grech, M.; Epele, L.; Kutschker, A.; Miserendino, M. Macrophyte regional patterns, metrics assessment and ecological integrity of isolated ponds at Austral Patagonia (Argentina). Sci. Total. Environ. 2020, 727, 138617. [Google Scholar] [CrossRef] [PubMed]
- Wraber, M. Pflanzengeographische Stellung und Gliedernung Sloweniens. Vegetatio 1969, 17, 176–199. [Google Scholar] [CrossRef]
- Sket, B. Oblikuje se današnje živalstvo. In Fauna of Slovenia; Sket, B., Gogala, M., Kuštor, V., Eds.; Tehniška založba Slovenije: Ljubljana, Slovenia, 2003; pp. 41–55. [Google Scholar]
- Illies, J. Limnofauna Europaea: Eine Zusammenstellung Aller Die Europäischen Binnengewässer bewohnenden Mehrzelligen Tierarten Mit Angaben Über Ihre Verbreitung Und Ökologie; Fischer: Stuttgart, Germany, 1978. [Google Scholar]
- Mršić, N. Biotic Diversity in Slovenia: Slovenia-the “Hot Spot” of Europe; Ministrstvo za Okolje in Prostor, Uprava RS za Varstvo Narave: Ljubljana, Slovenia, 1997; p. 129.
- Urbanic, G. Redelineation of European inland water ecoregions in Slovenia. Rev. Hydrobiol. 2008, 1, 17–25. [Google Scholar]
- Šraj-Kržič, N.; Germ, M.; Urbanc-Berčič, O.; Kuhar, U.; Janauer, G.A.; Gaberščik, A. The quality of the aquatic environment and macrophytes of karstic watercourses. Plant Ecol. 2007, 192, 107–118. [Google Scholar] [CrossRef]
- Hawes, I.; Riis, T.; Sutherland, D.; Flanagan, M. Physical Constraints to Aquatic Plant Growth in New Zealand Lakes. J. Publ. Title 2003, 41, 44–52. [Google Scholar]
- Van Geest, G.J.; Coops, H.; Roijackers, R.M.M.; Buijse, A.D.; Scheffer, M. Succession of aquatic vegetation driven by reduced water-level fluctuations in floodplain lakes. J. Appl. Ecol. 2005, 42, 251–260. [Google Scholar] [CrossRef]
- Alahuhta, J.; Lindholm, M.; Baastrup-Spohr, L.; García-Girón, J.; Toivanen, M.; Heino, J.; Murphy, K. Macroecology of macrophytes in the freshwater realm: Patterns, mechanisms and implications. Aquat. Bot. 2021, 168, 103325. [Google Scholar] [CrossRef]
- Jenačković, D.D.; Zlatković, I.D.; Lakušić, D.V.; Ranđelović, V.N. The assessment of seasonal variability in emergent macrophyte communities. Biologia 2016, 71, 287–297. [Google Scholar] [CrossRef]
- Landucci, F.; Řezníčková, M.; Šumberová, K.; Chytrý, M.; Aunina, L.; Biţă-Nicolae, C.; Bobrov, A.; Borsukevych, L.; Brisse, H.; ČARNI, A.; et al. WetVegEurope: A database of aquatic and wetland vegetation of Europe. Phytocoenologia 2015, 45, 187–194. [Google Scholar] [CrossRef]
- Hrvatin, M.; Tičar, J.; Zorn, M. Rocks and Tectonic Structure of Slovenia. In The Geography of Slovenia; Perko, D., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 23–34. [Google Scholar]
- Komac, B.; Pavšek, M.; Topole, M. Climate and Weather of Slovenia. In The Geography of Slovenia; Perko, D., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 71–89. [Google Scholar]
- Kolbezen, M.; Pristov, J.; Bat, M.; Klemenc, B.; Hrček, D. Surface Streams and Water Balance of Slovenia; Ministrstvo za Okolje in Prostor: Ljubljana, Slovenia, 1998; p. 98.
- Janauer, G.; Exler, N.; Anačkov, G.; Barta, V.; Berczik, Á.; Boža, P.; Dinka, M.; Georgiev, V.; Germ, M.; Holcar, M.; et al. Distribution of the Macrophyte Communities in the Danube Reflects River Serial Discontinuity. Water 2021, 13, 918. [Google Scholar] [CrossRef]
- Köhler, A.; Janauer, G.A. Zur Methodik der Untersuchung von aquatischen Makrophyten in Fließgewässern. Handbuch Angewandte Limnologie: Grundlagen-Gewässerbelastung-Restaurierung-Aquatische Ökotoxikologie-Bewertung-Gewässerschutz 2004, 8, 1–22. [Google Scholar] [CrossRef]
- Schneider, S.; Melzer, A. The Trophic Index of Macrophytes (TIM)—A New Tool for Indicating the Trophic State of Running Waters. Int. Rev. Hydrobiol. 2003, 88, 49–67. [Google Scholar] [CrossRef]
- Janauer, G.A.; Gaberscik, A.; Kvet, J.; Germ, M.; Exler, N. Macrophytes of the River Danube Basin; Academia: Praha, Czech Republic, 2018; p. 408. [Google Scholar]
- Kurtto, A.; Sennikov, A.; Lampinen, R. Atlas Florae Europaeae (AFE)—Distribution of Vascular Plants in Europe; The Committee for Mapping the Flora of Europeand Societas Biologica Fennica Vanamo: Helsinki, Finland, 2013; Available online: https://www.luomus.fi/en/atlas-florae-europaeae-afe-distribution-vascular-plants-europe (accessed on 9 February 2021).
- Schaumburg, J.; Schranz, C.; Foerster, J.; Gutowski, A.; Hofmann, G.; Meilinger, P.; Schneider, S.; Schmedtje, U. Ecological classification of macrophytes and phytobenthos for rivers in Germany according to the water framework directive. Limnologica 2004, 34, 283–301. [Google Scholar] [CrossRef] [Green Version]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin, Germany, 1964; p. 631. [Google Scholar]
- Lukács, B.A.; Tóthmérész, B.; Borics, G.; Várbíró, G.; Juhász, P.; Kiss, B.; Müller, Z.; G-Tóth, L.; Erős, T. Macrophyte diversity of lakes in the Pannon Ecoregion (Hungary). Limnologica 2015, 53, 74–83. [Google Scholar] [CrossRef]
- Pall, K.; Gecheva, G.; Soaru-Minea, A.; Lukacs, B.S.P. Intercalibration of the National Classifications of Ecological Status for Eastern Continental Lakes; Publications Office of the European Union: Rue Mercier, Luxembourg, 2018; p. 81. [Google Scholar]
- Germ, M.; Gaberščik, A.; Urbanc-Berčič, O. The wider environmental assessment of river ecosystems. Acta. Biol. Slov. 2000, 43, 13–19. [Google Scholar]
- Janauer, G.A.; Exler, N.; Schmidt-Mumm, U. The harmonised method for the macrophyte and habitat survey in the MIDCC-project: River Danube, floodplain waters and tributaries. In Macrophytes of the River Danube Basin; Janauer, G.A., Gaberščik, A., Květ, J., Germ, M., Exler, N., Eds.; Academia: Praha, Czech Republic, 2018; pp. 14–34. ISBN 978-80-200-2743-6. [Google Scholar]
- QGIS. Welcome to the QGIS project! Available online: http://www.qgis.org/ (accessed on 25 September 2020).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Zelnik, I. Vegetation of the Meadows from the Order Molinietalia W. Koch 1926 and Contact Sites in Slovenia. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 2005. [Google Scholar]
- Hrivnák, R.; Bubíková, K.; Oťaheľová, H.; Šumberová, K. Formalised classification of aquatic vegetation in Slovakia. Phytocoenologia 2019, 49, 107–133. [Google Scholar] [CrossRef]
- Landucci, F.; Tichý, L.; Šumberová, K.; Chytrý, M. Formalized classification of species-poor vegetation: A proposal of a consistent protocol for aquatic vegetation. J. Veg. Sci. 2015, 26, 791–803. [Google Scholar] [CrossRef]
- Šumberová, K. Vegetation of free floating aquatic plants (Lemnetea). In Vegetation of the Czech Republic. 3, Aquatic and Wetland Vegetation; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; pp. 43–99. [Google Scholar]
- Šumberová, K. Vegetation of aquatic plants rooted in the bottom (Potametea). In Vegetation of the Czech Republic. 3, Aquatic and Wetland Vegetation; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; pp. 100–247. [Google Scholar]
- Šumberová, K.; Lososová, Z. Vegetation of annual nitrophilous wetland herbs (Bidentetea tripartitae). In Vegetation of the Czech Republic. 3, Aquatic and Wetland Vegetation; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; pp. 347–384. [Google Scholar]
- Šumberová, K.; Hájková, P.; Chytrý, M.; Hroudová, Z.; Sádlo, J.; Hájek, M.; Hrivnák, R.; Navrátilová, J.; Hanáková, P.; Ekrt, L.; et al. Marsh vegetation (Phragmito-Magno-Caricetea). In Vegetation of the Czech Republic. 3, Aquatic and Wetland Vegetation; Chytrý, M., Ed.; Academia: Praha, Czech Republic, 2011; pp. 385–579. [Google Scholar]
- Zaliberová, M.; Škodová, I. Flood-meadows. In Plant Communities of Slovakia. 5. Grassland Vegetation; Hegedüšová, K., Škodová, I., Eds.; Veda: Bratislava, Slovakia, 2014. [Google Scholar]
- Landucci, F.; Šumberová, K.; Tichý, L.; Hennekens, S.; Aunina, L.; Biță-Nicolae, C.; Borsukevych, L.; Bobrov, A.; Čarni, A.; De Bie, E.; et al. Classification of the European marsh vegetation (Phragmito-Magnocaricetea) to the association level. Appl. Veg. Sci. 2020, 23, 297–316. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Verdonschot, P.F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Hudon, C.; Gagnon, P.; Jean, M. Hydrological factors controlling the spread of common reed (Phragmites australis) in theSt. Lawrence River (Québec, Canada). Écoscience 2005, 12, 347–357. [Google Scholar] [CrossRef]
- Ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Research Output of Wageningen University and Research Staff: Ithaca, NY, USA, 2002. [Google Scholar]
- Furnas, E.R. Available online: http://www.microcomputerpower.com/ (accessed on 2 March 2021).
- Fernández-Aláez, M.; García-Criado, F.; García-Girón, J.; Santiago, F.; Fernández-Aláez, C. Environmental heterogeneity drives macrophyte beta diversity patterns in permanent and temporary ponds in an agricultural landscape. Aquat. Sci. 2020, 82, 1–12. [Google Scholar] [CrossRef]
- Murphy, K.; Efremov, A.; Davidson, T.A.; Molina-Navarro, E.; Fidanza, K.; Betiol, T.C.C.; Chambers, P.; Grimaldo, J.T.; Martins, S.V.; Springuel, I.; et al. World distribution, diversity and endemism of aquatic macrophytes. Aquat. Bot. 2019, 158, 103127. [Google Scholar] [CrossRef]
- Maberly, S.C.; Haslam, S.M. River Plants of Western Europe: The Macrophytic Vegetation of the Watercourses of the European Economic Community. J. Ecol. 1988, 76, 1248. [Google Scholar] [CrossRef]
- Preston, C. Pondweeds of Great Britain and Ireland; Botanical Society of the British Isles: London, UK, 1995; p. 352. [Google Scholar]
- Brainard, A.S.; Luzadis, V.A.; Schulz, K.L. Drivers of species richness, biomass, and dominance of invasive macrophytes in temperate lakes. Biol. Invasions 2021, 23, 1069–1085. [Google Scholar] [CrossRef]
- Kuhar, U.; Germ, M.; Gaberščik, A. Habitat characteristics of an alien species Elodea canadensis in Slovenian watercourses. Hydrobiologia 2010, 656, 205–212. [Google Scholar] [CrossRef]
- O’Hare, M.T.; Baattrup-Pedersen, A.; Nijboer, R.; Szoszkiewicz, K.; Ferreira, T. Macrophyte communities of European streams with altered physical habitat. Hydrobiologia 2006, 566, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Janauer, G.; Dokulil, M. Macrophytes and Algae in Running Waters. In Biological Monitoring of Rivers: Applications and Perspectives; John, W., Ed.; Sons Ltd.: Chichester, UK, 2006; pp. 89–109. [Google Scholar]
- Li, G.; Hu, S.; Hou, H.; Kimura, S. Heterophylly: Phenotypic Plasticity of Leaf Shape in Aquatic and Amphibious Plants. Plants 2019, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Boulton, A.J.; Brock, M.A.; Robson, B.J.; Ryder, D.S.; Chambers, J.M.; Davis, J.A. Australian Freshwater Ecology: Processes and Management; Wiley-Blackwell: Hoboken, NJ, USA, 2014; p. 386. [Google Scholar]
- Nilsen, E.T.; Orcutt, D.M. Water limitation. In The Physiology of Plants under Stress. Abiotic Factors; John Wiley and Sons: New York, NY, USA, 1996; pp. 322–361. [Google Scholar]
- Brink, F.V.D.; Van Der Velde, G.; Bosman, W.; Coops, H. Effects of substrate parameters on growth responses of eight helophyte species in relation to flooding. Aquat. Bot. 1995, 50, 79–97. [Google Scholar] [CrossRef]
- Grimaldo, J.T.; O’Hare, M.T.; Kennedy, M.P.; Davidson, T.A.; Bonilla-Barbosa, J.; Santamaría-Araúz, B.; Gettys, L.; Martins, S.V.; Thomaz, S.M.; Murphy, K.J. Environmental drivers of freshwater macrophyte diversity and community composition in calcareous warm-water rivers of America and Africa. Freshw. Biol. 2017, 62, 1511–1527. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Kennedy, M.P.; Lang, P.; Grimaldo, J.T.; Martins, S.V.; Bruce, A.; Hastie, A.; Lowe, S.; Ali, M.M.; Sichingabula, H.; Dallas, H.; et al. Environmental drivers of aquatic macrophyte communities in southern tropical African rivers: Zambia as a case study. Aquat. Bot. 2015, 124, 19–28. [Google Scholar] [CrossRef]
- Alahuhta, J.; Lindholm, M.; Bove, C.P.; Chappuis, E.; Clayton, J.; De Winton, M.; Feldmann, T.; Ecke, F.; Gacia, E.; Grillas, P.; et al. Global patterns in the metacommunity structuring of lake macrophytes: Regional variations and driving factors. Oecologia 2018, 188, 1167–1182. [Google Scholar] [CrossRef] [Green Version]
- Grinberga, L. Environmental factors influencing the vegetation in middle-sized streams in Latvia. Ann. Bot. 2011, 1, 37–44. [Google Scholar] [CrossRef]
- Madsen, J.D.; Chambers, P.A.; James, W.F.; Koch, E.W.; Westlake, D.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 2001, 444, 71–84. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Mjelde, M.; Faafeng, B. Ceratophyllum demersum hampers phytoplankton development in some small Norwegian lakes over a wide range of phosphorus concentrations and geographical latitude. Freshw. Biol. 1997, 37, 355–365. [Google Scholar] [CrossRef]
- Hutchinson, G.E. The chemical ecology of three species of myriophyllum (Angiospermae, Haloragaceae) 1,2. Limnol. Oceanogr. 1970, 15, 1–5. [Google Scholar] [CrossRef]
- Grace, B.J.; Wetzel, R.G. The Production Biology of Eurasian Watermilfoil (Myriophyllum spicatum L.): A Review. J. Aquat. Plant Manag. 1978, 16, 1–11. [Google Scholar]
- Van Wijk, R. Ecological studies on Potamogeton pectinatus L. I. General characteristics, biomass production and life cycles under field conditions. Aquat. Bot. 1988, 31, 211–258. [Google Scholar] [CrossRef]
- Germ, M.; Kuhar, U.; Gaberščik, A. Abundance and Diversity of Taxa Within the Genus Potamogeton in Slovenian Watercourses. In Natural and Constructed Wetlands; Springer International Publishing: Cham, Switzerland, 2016; pp. 283–291. [Google Scholar]
- Dolinar, N.; Regvar, M.; Abram, D.; Gaberščik, A. Water-level fluctuations as a driver of Phragmites australis primary productivity, litter decomposition, and fungal root colonisation in an intermittent wetland. Hydrobiologia 2015, 774, 69–80. [Google Scholar] [CrossRef]
- Gaberščik, A.; Grašič, M.; Abram, D.; Zelnik, I. Water Level Fluctuations and Air Temperatures Affect Common Reed Habitus and Productivity in an Intermittent Wetland Ecosystem. Water 2020, 12, 2806. [Google Scholar] [CrossRef]
- Martinčič, A. Pteridophytes and Spermatophytes. In Vanishing Lake–Monography on lake Cerknica (Slovenian with English Summary); Gaberščik, A., Ed.; Društvo ekologov Slovenije: Ljubljana, Slovenia, 2002; pp. 76–80. [Google Scholar]
- Jenačković, D.D.; Zlatković, I.D.; Lakušić, D.V.; Ranđelović, V.N. Macrophytes as bioindicators of the physicochemical characteristics of wetlands in lowland and mountain regions of the central Balkan Peninsula. Aquat. Bot. 2016, 134, 1–9. [Google Scholar] [CrossRef]
- Clevering, A.O.; Brix, H.; Lukavská, J. Geographic variation in growth responses in Phragmites australis. Aquat. Bot. 2001, 69, 89–108. [Google Scholar] [CrossRef]
- Píšová, S.; Fér, T. Intraspecific differentiation of Sparganium erectum in the Czech Republic. PRESLIA 2020, 92. [Google Scholar] [CrossRef]
- Landucci, F.; Republic, C.; Gigante, D.; Venanzoni, R.; Chytrý, M. Wetland vegetation of the class Phragmito-Magno-Caricetea in centralItaly. Phytocoenologia 2013, 43, 67–102. [Google Scholar] [CrossRef] [Green Version]
- Misson, G.; Macor, A.; Boscutti, F.; Casolo, V. Ecological characterisation of Hippuris vulgaris populations growing in spring Water Rivers. Phyton (Horn) 2016, 56, 209–224. [Google Scholar] [CrossRef]






| Variable | No. of Taxa | S-W | Abundance |
|---|---|---|---|
| Distance from the source | −0.314 *** | −0.304 *** | −0.215 *** |
| Altitude | 0.383 *** | 0.363 *** | 0.175 *** |
| Slope | −0.141 *** | −0.132 *** | −0.167 *** |
| Land use | 0.084 ** | 0.097 ** | 0.115 *** |
| Width of riparian zone | ns | ns | ns |
| Completeness of riparian zone | 0.123 *** | 0.131 *** | 0.133 *** |
| Vegetation of riparian zone | 0.072 * | 0.078 * | 0.131 *** |
| Retention structures | 0.188 *** | 0.169 *** | 0.104 ** |
| Sediment deposition | 0.196 *** | 0.206 *** | 0.184 *** |
| Riverbed bottom | 0.207 *** | 0.180 *** | 0.202 *** |
| Flow dynamics | 0.120 *** | 0.129 *** | 0.121 *** |
| Presence of detritus | 0.216 *** | 0.189 *** | 0.192 *** |
| Current velocity | −0.365 *** | −0.322 *** | −0.386 *** |
| Presence of filamentous algae | −0.205 *** | −0.191 *** | ns |
| Formal Definition | ||||
|---|---|---|---|---|
| Cluster Number | N | Plant Communities | Coverage (%) of Dominant Taxa; Subdominant | NOT |
| 1 | 6 | Ceratophylletum demersi Corillion 1957 | Ceratophyllum demersum >50% | Potamogeton crispus >25% Potamogeton natans >25% Potamogeton nodosus >25% Ceratophyllum submersum >25% Nuphar luteum >25% Phragmites australis >25% Sagittaria sagittifolia >25% Sparganium erectum >25% |
| 2 | 31 | Potametum denso-nodosi de Bolós 1957 | Potamogeton nodosus >25%; codominant C. demersum | |
| 3 | 10 | Potametum denso-nodosi de Bolós 1957 | Potamogeton nodosus >25%; subdominant C. demersum | Sagittaria sagittifolia > 25% Sparganium emersum >25% |
| 4 | 10 | Potametum denso-nodosi de Bolós 1957 | Potamogeton nodosus >25% | |
| 5 | 5 | Myriophylletum verticillati Gaudet ex Šumberová in Chytrý 2011 | Myriophyllum verticillatum >25% | |
| 6 | 31 | Potamo pectinati-Myriophylletum spicati Rivas Goday 1964 | Myriophyllum spicatum >50% | Butomus umbellatus >25% Nuphar luteum >25% Sparganium emersum >25% |
| 7 | 6 | Potamo pectinati-Myriophylletum spicati Rivas Goday 1964 | Myriophyllum spicatum >50% | Potamogeton pectinatus |
| 8 | 8 | Potametum pectinati Carstensen ex Hilbig 1971 | Potamogeton pectinatus >50% | Zannichellia palustris >5% |
| 9 | 7 | Polygonetum hydropiperis Passarge 1965 | Polygonum hydropiper/mite >50% codom.: Myriophyllum spicatum, Phalaris arundinacea | Chenopodium glaucum Ranunculus sceleratus Urtica dioica >25% |
| 10 | 12 | Phragmitetum australis Savič 1926 | Phragmites australis >50% | |
| 11 | 26 | Phalaridetum arundinaceae Libbert 1931 | Phalaris arundinacea >25% AND (groups Bidens frondosa/Cirsium oleraceum/Urtica dioica) | |
| 12 | 6 | Rumici crispi-Agrostietum stoloniferae Moor 1958 | Rorippa sylvestris AND Agrostis stolonifera agg. >25% OR Bidens tripartita >50% | |
| 13 | 109 | Community with Myriophyllum spicatum | dominant Myriophyllum spicatum | low dominance (15%)–Phalaris arundinacea, Potamogeton nodosus |
| 14 | 4 | Oenantho aquaticae-Rorippetum amphibiae Lohmeyer 1950 | Rorippa amphibia >25%; Alisma plantago-aquatica | Glyceria maxima cover >25% Phalaris arundinacea>25% Phragmites australis>25% Sagittaria sagittifolia>25% Schoenoplectus lacustris >25%Sparganium erectum >25% |
| 15 | 4 | Glycerio notatae-Veronicetum beccabungae Landucci et al. 2020 | Veronica beccabunga | |
| 16 | 5 | Alopecuro-Alismatetum plantaginis-aquaticae Bolbrinker 1984 | Alisma plantago-aquatica >25% | Alisma lanceolatum >25% Glyceria maxima >25% Sparganium emersum >25% Sparganium erectum >25% |
| 17 | 226 | species-poorest. abundance lowest stretches | Phalaris arundinacea. Lytrum salicaria, Myosotis scorpioides | |
| 18 | 30 | Phragmitetum australis Savič 1926 | Phragmites australis | low dominance (22%); Nuphar luteum |
| 19 | 5 | Mentho aquaticae-Oenanthetum fistulosae ass. nova | Oenanthe fistulosa >50% | |
| 20 | 27 | Potametum crispi von Soó 1927 | Potamogeton crispus >25% | Nuphar luteum>25% Potamogeton natans>25% Phragmites australis>25% |
| 21 | 32 | Potamo crispi-Ranunculetum trichophylli Imchenetzky 1926 | Ranunculus trichophyllus >25%; Potamogeton crispus | Glyceria notata>25% Juncus articulatus>25% |
| 22 | 23 | Potametum perfoliati Miljan 1933 | Potamogeton perfoliatus >25% | - |
| 23 | 18 | Glycerio-Sparganietum neglecti Koch 1926 | Sparganium erectum agg. >25% | Typha latifolia >25% |
| 24 | 87 | Potamo pectinati-Myriophylletum spicati Rivas Goday 1964 | dominant Myriophyllum spicatum | |
| 25 | 18 | Schoenoplectetum lacustris Chouard 1924 | Schoenoplectus lacustris >25% | - |
| 26 | 13 | Phalaridetum arundinaceae Libbert 1931 | Phalaris arundinacea >25% AND (groups Bidens frondosa/Cirsium oleraceum/Urtica dioica) | |
| 27 | 15 | Nymphaeo albae-Nupharetum luteae Nowiński 1927 | Nuphar luteum >25% | Nymphaea alba >5% Typha latifolia>25% |
| 28 | 7 | Beruletum erectae Roll 1938 | Berula erecta >5%; co-dominant Callitriche spp. | Potamogeton perfoliatus, Sparganium emersum |
| 29 | 38 | Beruletum erectae Roll 1938 | Berula erecta >5%; subdom. Callitriche spp. | |
| 30 | 19 | Sagittario sagittifoliae-Sparganietum emersi Tüxen 1953 | Sagittaria sagittifolia >25% OR Sparganium emersum >25% | Butomus umbellatus >25% Glyceria maxima >25% Potamogeton gramineus >25% Rorippa amphibia cover >25% Sparganium erectum >25% |
| 31 | 11 | Beruletum erectae Roll 1938 | Berula erecta >5% | |
| 32 | 18 | Potametum natantis Hild 1959 | Potamogeton natans >25% | Callitriche palustris s. l. >25% Eleocharis palustris >25% Glyceria fluitans >25% Hippuris vulgaris >25% Nuphar luteum >25% Phragmites australis >25% Potamogeton nodosus >25% Sparganium emersum >25% Sparganium erectum >25% |
| 33 | 4 | Eleocharito palustris-Hippuridetum vulgaris Passarge 1964 | Hippuris vulgaris >25% | - |
| 34 | 35 | Elodeetum canadensis Nedelcu 1967 | Elodea canadensis >50% | Equisetum fluviatile >25% Potamogeton natans >25% Schoenoplectus lacustris >25% Sparganium erectum >25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelnik, I.; Kuhar, U.; Holcar, M.; Germ, M.; Gaberščik, A. Distribution of Vascular Plant Communities in Slovenian Watercourses. Water 2021, 13, 1071. https://doi.org/10.3390/w13081071
Zelnik I, Kuhar U, Holcar M, Germ M, Gaberščik A. Distribution of Vascular Plant Communities in Slovenian Watercourses. Water. 2021; 13(8):1071. https://doi.org/10.3390/w13081071
Chicago/Turabian StyleZelnik, Igor, Urška Kuhar, Matej Holcar, Mateja Germ, and Alenka Gaberščik. 2021. "Distribution of Vascular Plant Communities in Slovenian Watercourses" Water 13, no. 8: 1071. https://doi.org/10.3390/w13081071
APA StyleZelnik, I., Kuhar, U., Holcar, M., Germ, M., & Gaberščik, A. (2021). Distribution of Vascular Plant Communities in Slovenian Watercourses. Water, 13(8), 1071. https://doi.org/10.3390/w13081071