Biological Desulfurization of Tannery Effluent Using Hybrid Linear Flow Channel Reactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Cultures for Inoculation of Hybrid Linear Channel Reactors
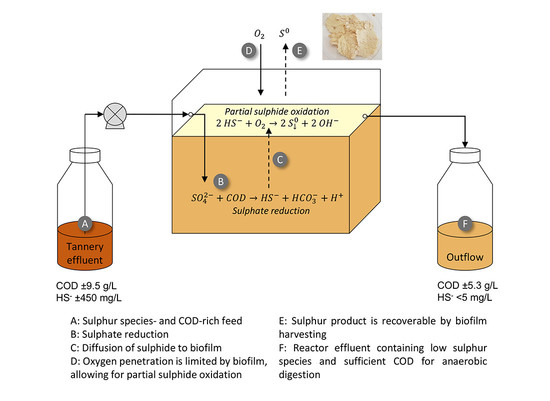
2.2. Tannery Wastewater
2.3. Set-Up and Operation of Hybrid Linear Flow Channel Reactors (HLFCR)
2.4. Batch Anaerobic Digestion
2.5. Sampling
2.5.1. Hybrid Linear Flow Channel Reactors
2.5.2. Anaerobic Digesters
2.6. Analytical Methods
3. Results and Discussion
3.1. Operation and Performance of Hybrid Linear Channel Reactors for Removal of Sulfur Species
3.2. Sulfur Species Removal Rates
3.3. Floating Sulfur Biofilm Formation and Sulfur Recovery
3.4. COD Utilization
3.5. Batch Anaerobic Digestion of Tannery Effluent before and after Pretreatment in Hybrid Linear Channel Reactors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sabumon, P.C. Perspectives on Biological Treatment of Tannery Effluent. Adv. Recycl. Waste Manag. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Mpofu, A.B.; Oyekola, O.O.; Welz, P.J. Anaerobic treatment of tannery wastewater in the context of a circular bioeconomy for developing countries. J. Clean. Prod. 2021, 296, 126490. [Google Scholar] [CrossRef]
- Swartz, C.D.; Jackson-Moss, C.; Rowswell, R.A.; Mpofu, A.B.; Welz, P.J. Water and Wastewater Management in the Tanning and Leather Finishing Industry: NATSURV 10, 2nd ed.; Report TT 713/17; Water Research Commission: Pretoria, South Africa, 2017. [CrossRef]
- Oyekola, O.; van Hille, R.P.; Harrison, S.T. Kinetic analysis of biological sulphate reduction using lactate as carbon source and electron donor: Effect of sulphate concentration. Chem. Eng. Sci. 2010, 65, 4771–4781. [Google Scholar] [CrossRef]
- Selvaraj, H.; Aravind, P.; George, H.S.; Sundaram, M. Removal of sulfide and recycling of recovered product from tannery lime wastewater using photoassisted-electrochemical oxidation process. J. Ind. Eng. Chem. 2019, 83, 164–172. [Google Scholar] [CrossRef]
- Yang, J.H. Hydrogen sulfide removal technology: A focused review on adsorption and catalytic oxidation. Korean J. Chem. Eng. 2021, 38, 674–691. [Google Scholar] [CrossRef]
- Mpofu, A.B.; Oyekola, O.O.; Welz, P.J. Co-digestion of tannery waste activated sludge with slaughterhouse sludge to improve organic biodegradability and biomethane generation. Process. Saf. Environ. Prot. 2020, 131, 235–245. [Google Scholar] [CrossRef]
- Kibangou, V.A.; Lilly, M.; Mpofu, A.B.; de Jonge, N.; Oyekola, O.O.; Welz, P.J. Sulfate-reducing and methanogenic microbial community responses during anaerobic digestion of tannery effluent. Bioresour. Technol. 2021, 126308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cheng, H.; Chen, F.; Zhang, Y.; Xu, X.; Huang, C.; Chen, C.; Liu, W.; Ding, C.; Li, Z.; et al. Enhanced methane production by alleviating sulfide inhibition with a microbial electrolysis coupled anaerobic digestion reactor. Environ. Int. 2020, 136, 105503. [Google Scholar] [CrossRef] [PubMed]
- Theuerl, S.; Klang, J.; Hülsemann, B.; Mächtig, T.; Hassa, J. Microbiome Diversity and Community-Level Change Points within Manure-Based Small Biogas Plants. Microorganisms 2020, 8, 1169. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Puhakka, J.A. Sulfate Reduction Based Bioprocesses for the Treatment of Acid Mine Drainage and the Recovery of Metals. Eng. Life Sci. 2007, 7, 541–564. [Google Scholar] [CrossRef]
- Lens, P.N.L.; Visser, A.; Janssen, A.J.H.; Pol, L.H.; Lettinga, G. Biotechnological Treatment of Sulfate-Rich Wastewaters. Crit. Rev. Environ. Sci. Technol. 1998, 28, 41–88. [Google Scholar] [CrossRef]
- Midha, V.; Dey, A. Biological Treatment of Tannery Wastewater for Sulphide Removal. Int. J. Chem. Sci. 2008, 6. Available online: https://www.semanticscholar.org/paper/BIOLOGICAL-TREATMENT-OF-TANNERY-WASTEWAT-ER-FOR-Midha-Dey/4556f7a2140af2d59e7f4820ed23309ad2b62a8d (accessed on 25 November 2021).
- Mahmood, Q.; Zheng, P.; Cai, J.; Hayat, Y.; Hassan, M.J.; Wu, D.-L.; Hu, B.-L. Sources of sulfide in waste streams and current biotechnologies for its removal. J. Zhejiang Univ.-Sci. A 2007, 8, 1126–1140. [Google Scholar] [CrossRef]
- Alesia, F.D. Optimization of Elemental Sulphur Recovery during an Acid Mine Water Treatment. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2015. [Google Scholar] [CrossRef]
- Marais, T.; Huddy, R.; Harrison, S.; van Hille, R. Demonstration of simultaneous biological sulphate reduction and partial sulphide oxidation in a hybrid linear flow channel reactor. J. Water Process. Eng. 2020, 34, 101143. [Google Scholar] [CrossRef]
- Mooruth, N. An Investigation towards Passive Treatment Solutions for the Oxidation of Sulphide and Subsequent Removal of Sulphur from Acid Mine Water. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2013. Available online: http://hdl.handle.net/11427/5437 (accessed on 25 November 2021).
- Mpofu, A.; Kibangou, V.; Kaira, W.; Oyekola, O.; Welz, P. Anaerobic Co-Digestion of Tannery and Slaughterhouse Wastewater for Solids Reduction and Resource Recovery: Effect of Sulfate Concentration and Inoculum to Substrate Ratio. Energies 2021, 14, 2491. [Google Scholar] [CrossRef]
- Hobo, A.; Schuman, E.; van Eekert, M. Testen Vergisten Kippenmest; Conceptrapport No. 18–205; LEAF: Wageningen, The Netherlands, 2019. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, CO, USA; Water Environment Federation (WEF): Alexandria, VA, USA, 2012. [Google Scholar] [CrossRef] [Green Version]
- Omil, F.; Lens, P.; Pol, L.H.; Lettinga, G. Effect of upward velocity and sulphide concentration on volatile fatty acid degradation in a sulphidogenic granular sludge reactor. Process. Biochem. 1996, 31, 699–710. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; WILEY-VCH Verlag: Weinheim, Germany, 2008; pp. 1–443. [Google Scholar] [CrossRef]
- Boshoff, G.; Duncan, J.; Rose, P. Tannery effluent as a carbon source for biological sulphate reduction. Water Res. 2004, 38, 2651–2658. [Google Scholar] [CrossRef]
- Xu, X.-J.; Chen, C.; Wang, A.-J.; Fang, N.; Yuan, Y.; Ren, N.-Q.; Lee, D.-J. Enhanced elementary sulfur recovery in integrated sulfate-reducing, sulfur-producing rector under micro-aerobic condition. Bioresour. Technol. 2012, 116, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Sabumon, P. Development of enhanced sulphidogenesis process for the treatment of wastewater having low COD/SO42− Ratio. J. Hazard. Mater. 2008, 159, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Moosa, S.; Nemati, M.; Harrison, S. A kinetic study on anaerobic reduction of sulphate, Part I: Effect of sulphate concentration. Chem. Eng. Sci. 2002, 57, 2773–2780. [Google Scholar] [CrossRef]
- Kleinjan, W.E.; de Keizer, A.; Janssen, A.J.H. Kinetics of the Reaction between Dissolved Sodium Sulfide and Biologically Produced Sulfur. Ind. Eng. Chem. Res. 2004, 44, 309–317. [Google Scholar] [CrossRef]
- Lu, H.; Ekama, G.A.; Wu, D.; Feng, J.; van Loosdrecht, M.C.M.; Chen, G.-H. SANI® process realizes sustainable saline sewage treatment: Steady state model-based evaluation of the pilot-scale trial of the process. Water Res. 2012, 46, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Nielsen, P.; Jones, W.; Characklis, W. Sulfide product inhibition of Desulfovibrio desulfuricans in batch and continuous cultures. Water Res. 1995, 29, 571–578. [Google Scholar] [CrossRef]
- Bajpai, P. Chapter 2: Basics of Anaerobic Digestion Process. In Anaerobic Technology in Pulp and Paper Industry; Springer Briefs in Applied Sciences and Technology: Singapore, 2017; pp. 7–12. [Google Scholar] [CrossRef]
- Ahring, B.K.; Murali, N.; Srinivas, K. Fermentation of Cellulose with a Mixed Microbial Rumen Culture with and without Methanogenesis. Ferment. Technol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Wang, W.; Wachemo, A.C.; Zou, D. Effects of adding osmoprotectant on anaerobic digestion of kitchen waste with high level of salinity. J. Biosci. Bioeng. 2019, 128, 723–732. [Google Scholar] [CrossRef] [PubMed]





| Parameter | RAW | PARTIALLY TREATED | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| pH | 10.24 | 1.6 | 7.81 | 0.52 |
| EC (mS/cm) | 32.01 | 2.2 | 31.5 | 1.59 |
| ORP (mV) | −547 | 61.5 | −434 | 21.6 |
| TOC (mg/L) | 6116 | 1875 | 886 | 253 |
| COD (mg/L) | 28,169 | 3665 | 4968 | 2940 |
| BOD (mg/L) | 6200 | 812 | 1539 | 520 |
| VOAt (mg/L AAE) | 2920 | 718 | 1041 | 647 |
| Protein (mg/L) | 2875 | 1024 | 310.6 | 83.9 |
| TN (mg/L) | 1258 | 215 | 679 | 138 |
| TAN (mg/L NH3-N) | 301 | 286 | 350 | 235 |
| NO3− (mg/L) | 70.8 | 25.0 | 40.5 | 23.6 |
| NO2− (mg/L) | 5.1 | 5.2 | 2.35 | 2.63 |
| PO43− mg/L | 0 | 0 | 1.21 | 1.96 |
| SO42− (mg/L) | 1951 | 574 | 3687 | 383 |
| HS− (mg/L) | 699 | 114 | 83 | 76 |
| Cl− (mg/L) | 7744 | 460 | 7713 | 325 |
| TS (g/L) | 36.1 | 5.8 | 17.96 | 3.23 |
| TVS (g/L) | 13.1 | 3.3 | 1.93 | 0.47 |
| K (mg/L) | 95.7 | 31.1 | 100.3 | 19.5 |
| Na (mg/L) | 6412 | 571 | 6225 | 276 |
| Fe (mg/L) | 0.11 | 0.08 | 0.19 | 0.12 |
| Ca (mg/L) | 692 | 482 | 230.9 | 58.5 |
| Mg (mg/L) | 120 | 138 | 220.7 | 27.6 |
| Mn (mg/L) | 0.50 | 0.41 | 15.14 | 6.92 |
| Zn (mg/L) | 0.50 | 0.33 | 0.19 | 0.12 |
| Cr (mg/L) | 0.09 | 0.05 | 0.20 | 0.08 |
| Alk (mg/L CaCO3) | 3256 | 907 | 1999 | 385 |
| COD:SO42− | 15.4 | 3.5 | 1.4 | 0.76 |
| TVS:TS | 0.36 | 0.03 | 0.11 | 0.02 |
| BOD:COD | 0.23 | 0.05 | 0.37 | 0.14 |
| C:N | 5.1 | 2.2 | 1.3 | 0.21 |
| VOA:Alk | 0.94 | 0.27 | 0.48 | 0.24 |
| COD:TVS | 2.2 | 0.52 | 2.5 | 0.93 |
| Day | 0 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | 58 | 64 | 70 | 76 | 82 | 88 | 94 | 100 | 120 | 140 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLFCR1 | ||||||||||||||||||||||||
| Batch | ||||||||||||||||||||||||
| 1 day HRT | ||||||||||||||||||||||||
| 2 day HRT | ||||||||||||||||||||||||
| 4 day HRT | ||||||||||||||||||||||||
| HLFCR2 | ||||||||||||||||||||||||
| Batch | ||||||||||||||||||||||||
| 4 day HRT |
| Methane Yield | Controls | Pretreated Tannery Wastewater | |||
|---|---|---|---|---|---|
| Acetate | Acetate + NaCl | Undiluted | 50% Dilution | 50% Dilution + Acetate | |
| mL CH4/g CODconsumed | 342 | 208 | 38.4 | 130 | 214 |
| % of biogas | 69.7 | 42.45 | 13.7 | 32.5 | 46.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horn, E.J.; Oyekola, O.O.; Welz, P.J.; van Hille, R.P. Biological Desulfurization of Tannery Effluent Using Hybrid Linear Flow Channel Reactors. Water 2022, 14, 32. https://doi.org/10.3390/w14010032
Horn EJ, Oyekola OO, Welz PJ, van Hille RP. Biological Desulfurization of Tannery Effluent Using Hybrid Linear Flow Channel Reactors. Water. 2022; 14(1):32. https://doi.org/10.3390/w14010032
Chicago/Turabian StyleHorn, Emma Jane, Oluwaseun O. Oyekola, Pamela Jean Welz, and Robert Paul van Hille. 2022. "Biological Desulfurization of Tannery Effluent Using Hybrid Linear Flow Channel Reactors" Water 14, no. 1: 32. https://doi.org/10.3390/w14010032
APA StyleHorn, E. J., Oyekola, O. O., Welz, P. J., & van Hille, R. P. (2022). Biological Desulfurization of Tannery Effluent Using Hybrid Linear Flow Channel Reactors. Water, 14(1), 32. https://doi.org/10.3390/w14010032