Removal of Trichloroethylene from Water by Bimetallic Ni/Fe Nanoparticles
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Synthesis of Ni-nZVI Nanohybrids
2.3. Materials Characterization
2.4. TCE Degradation
3. Results and Discussion
3.1. X-ray Diffractometry
3.2. Morphology
3.3. X-ray Photoelectron Spectrometer
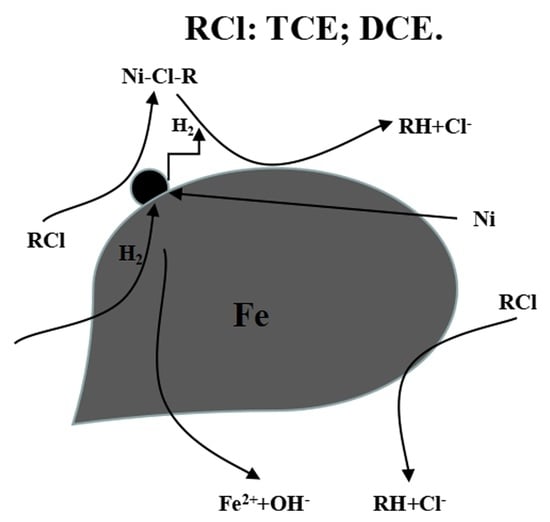
3.4. Morphology TCE Degradation by Ni-nZVI and nZVI
3.5. Kinetic Analysis of TCE Degradation with Different Ni Contents of Ni-nZVI
3.6. pH DO Change Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ remediation: A review of the benefits and potential risks. Environ. Health Perspect. 2009, 117, 1823–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FreirIa-Gádara, M.J.; Lorenzo-Perreira, R.A.; Alvarez-Devesa, A.; Bermejo, F. Occurrence of halogenated hydrocarbons in the water supply of ifferent cities of alicia (Spain). Environ Technol. 1992, 13, 437–447. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Y.; Shao, B. Reinvestigation of the oxidation of organic contaminants by Fe(VI): Kinetics and effects of water matrix constituents. J. Hazard Mat. 2022, 430, 128421. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Kang, S.W.; Ahn, S.; Woo, M.; Yang, S.K. Fe (0) nanoparticles for nitrate reduction: Stability, reactivity, and transformation. Environ. Sci. Technol. 2006, 40, 5514–5519. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Kim, T.; Ahn, J.-Y.; Hwang, K.-Y.; Park, J.-Y.; Lim, T.-T. Aging characteristics and reactivity of two types of nanoscale zero-valent iron particles (FeBH and FeH2) in nitrate reduction. Chem. Eng. J. 2012, 197, 16–23. [Google Scholar] [CrossRef]
- Chi, H.N.; Mai, L.T.; Tran, T.; Juang, R.S. Efficient removal of antibiotic oxytetracycline from water by Fenton-like reactions using reduced graphene oxide-supported bimetallic Pd/nZVI nanocomposites. J. Taiwan Inst. Chem E. 2021, 119, 80–89. [Google Scholar]
- Yu, Y.; Zheng, H.; Deng, D.; Ju, Y.; Hui, L. Synthesis of millimeter-scale sponge Fe/Cu bimetallic particles removing TBBPA and insights of degradation mechanism. Chem. Eng. J. 2017, 325, 279–288. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yue, P.L.; Chen, G. Catalytic dechlorination of chlorophenols in water by palladium/iron. Water Res. 2001, 35, 1887–1890. [Google Scholar] [CrossRef] [Green Version]
- Fennelly, J.P.; Roberts, A.L. Reaction of 1,1,1-trichloroethane with zero-valent metals and bimetallic reductants. Environ. Sci. Technol. 1998, 32, 1980–1988. [Google Scholar] [CrossRef]
- Elliott, D.W.; Zhang, W.X. Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef]
- Schrick, B.; Blough, J.L.; Jones; Daniel, A.; Mallouk, T.E. Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic Nickel-Iron nanoparticles. Chem Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Ii, C.J.C.; Rao, P.; Annable, M. Degradation of perchloroethylene in cosolvent solutions by zero-valent iron. J. Hazard. Mater. 2003, 96, 65–78. [Google Scholar]
- Jian, X.; Dozier, A.; Bhattacharyya, D. Synthesis of nanoscale bimetallic particles in polyelectrolyte membrane matrix for reductive transformation of halogenated organic compounds. J. Nanopart. Res. 2005, 7, 449–467. [Google Scholar]
- Yamashita, H.; Yoshikawa, M.; Funabiki, T.; Yoshida, S. Catalysis by amorphous metal alloys. J. Catal. 1986, 99, 375–382. [Google Scholar] [CrossRef]
- Wang, C.B.; Zhang, W.X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ. Sci. Technol. 1997, 31, 9602–9607. [Google Scholar] [CrossRef]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr (VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef]
- Fan, M.; Li, T.; Hu, J.; Cao, R.; Wu, Q.; Wei, X. Synthesis and characterization of reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites used for Pb (II) removal. Materials 2016, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Vipulanandan, C. Microemulsion and solution approaches to nanoparticle iron production for degradation of trichloroethylene. Colloids Surf. A Physicochem. Eng. Asp. 2013, 223, 103–112. [Google Scholar] [CrossRef]
- Pandey, D.; Ray, K.; Bhardwaj, R.; Bojja, S.; Chary KV, R.; Deo, G. Promotion of unsupported nickel catalyst using iron for CO2 methanation. Int. J. Hydrog. Energy. 2018, 43, 4987–5000. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Effect of support on the catalytic activity of supported Ni–Fe catalysts for the CO2 methanation reaction. J. Ind. Eng. Chem. 2016, 33, 99–107. [Google Scholar] [CrossRef]
- Wu, L.; Ritchie, S.M. Removal of trichloroethylene from water by cellulose acetate supported bimetallic Ni/Fe nanoparticles. Chemosphere 2006, 63, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Gao, B.; Wang, X.; Yin, X.; Feng, K. The sorptive and reductive capacities of biochar supported nanoscaled zero-valent iron (nZVI) in relation to its crystallite size. Chemosphere 2017, 186, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.; Meißner, T.; Potthoff, A.; Bleyl, S.; Georgi, A.; Mackenzie, K.; Trabitzsch, R.; Werban, U.; Oswald, S.E. A field investigation on transport of carbon-supported nanoscale zero-valent iron (nZVI) in groundwater. J. Hydrol. 2015, 181, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, L.; Wen, N.; Deng, D. Critical roles of sulfidation solvent in controlling surface properties and the dechlorination reactivity of S-nZVI. J. Hazard. Mater. 2021, 417, 126014. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Hadjipanayis, G.C.; Sorensen, C.M.; Klabunde, K.J. Exchange anisotropy in oxide passivated Co fine particles. J. Appl. Phys. 1993, 73, 6964–6966. [Google Scholar] [CrossRef]
- Yao, Y.H.; Huang, S.M.; Zhou, W. Highly dispersed core-shell iron nanoparticles decorating onto graphene nanosheets for superior Zn (II) wastewater treatment. Environ. Sci. Pollut. Res. 2018, 26, 806–815. [Google Scholar] [CrossRef]
- Ji, S.T.; Giang, B.L.; Sharanjit, S.; Duc, T.Q.; Thanh, H.; Huu, H. Methane bi-reforming over boron-doped Ni/SBA-15 catalyst: Longevity evaluation. Int. J. Hydrog. Energy. 2018, 44, 20839–20850. [Google Scholar]
- Wang, Z.; Dong, P.; Sun, Z.; Sun, C.; Bu, H.Y.; Han, J. NH2-Ni-MOF electrocatalysts with tunable size/morphology for ultrasensitive C-reactive protein detection via an aptamer binding induced DNA walker—Antibody sandwich assay. J. Mater. Chem B. 2018, 6, 2426–2431. [Google Scholar] [CrossRef]
- Tao, L.; Ai, Z.; Zhang, L. Fe@Fe2O3 core—Shell nanowires as iron reagent. 4. Sono—Fenton degradation of pentachlorophenol and the mechanism analysis. J. Phys. Chem. C. 2008, 112, 8675–8681. [Google Scholar]
- Antony, J.; Meera, V.; Raphael, V.P. Investigations on the capacity and mechanism of iron uptake by nano zero-valent iron particles. B Mater. Sci. 2021, 44, 1–11. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Huang, Y.; Kang, W.; Wang, Z.; Zheng, H. Roles of hydroxyl and carbonate radicals in bisphenol a degradation via a nanoscale zero-valent iron/percarbonate system: Influencing factors and mechanisms. RSC Adv. 2021, 11, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Chikate, R.C.; Rode, C.V.; Paknikar, K.M. Iron-nickel bimetallic nanoparticles for reductive degradation of azo dye Orange G in aqueous solution. Appl. Catal. B 2008, 79, 270–278. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, L.; Li, Y.; Han, B.; Feng, Q. Rapid degradation of carbon tetrachloride by microscale Ag/Fe bimetallic particles. Int. J. Environ. Res. Public Health 2021, 18, 2124. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Z.; Wang, Z.; Wang, Z.L.; Bush, R. Highly efficient and rapid removal of arsenic (III) from aqueous solutions by nanoscale zero-valent iron supported on a zirconium 1,4-dicarboxybenzene metal—Organic framework (UiO-66 MOF). RSC Adv. 2019, 9, 39475–39487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S.; Yang, N.; Zhuang, X.; Ren, L.; Zhan, J. Montmorillonite immobilized Fe/Ni bimetallic prepared by dry in-situ hydrogen reduction for the degradation of 4-Chlorophenlo. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ying, G.A.; Fw, A.; Yan, W.A.; Rn, B.; Zca, B. Comparison of degradation mechanisms of microcystin-LR using nanoscale zero-valent iron (nZVI) and bimetallic Fe/Ni and Fe/Pd nanoparticles. Chem. Eng. J. 2016, 285, 459–466. [Google Scholar]






| The Reactants | Reaction Time (h) | Degradation Rate (%) | Correlation Coefficient | Reaction Rate K (h−1) |
|---|---|---|---|---|
| nZVI | 1 | 47.85 | 0.98 | 0.675 |
| 0.5% Ni-nZVI | 1 | 60.01 | 0.99 | 0.909 |
| 3% Ni-nZVI | 1 | 89.28 | 0.99 | 1.663 |
| 5% Ni-nZVI | 1 | 98.18 | 0.99 | 2.358 |
| 10% Ni-nZVI | 1 | 72.91 | 0.99 | 1.250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, M.; Zhao, J. Removal of Trichloroethylene from Water by Bimetallic Ni/Fe Nanoparticles. Water 2022, 14, 1616. https://doi.org/10.3390/w14101616
Liu X, Wu M, Zhao J. Removal of Trichloroethylene from Water by Bimetallic Ni/Fe Nanoparticles. Water. 2022; 14(10):1616. https://doi.org/10.3390/w14101616
Chicago/Turabian StyleLiu, Xiaonan, Minghong Wu, and Jian Zhao. 2022. "Removal of Trichloroethylene from Water by Bimetallic Ni/Fe Nanoparticles" Water 14, no. 10: 1616. https://doi.org/10.3390/w14101616
APA StyleLiu, X., Wu, M., & Zhao, J. (2022). Removal of Trichloroethylene from Water by Bimetallic Ni/Fe Nanoparticles. Water, 14(10), 1616. https://doi.org/10.3390/w14101616