Impact of Livestock Farming on Nitrogen Pollution and the Corresponding Energy Demand for Zero Liquid Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manure Quantification for Nitrogen Recovery per kg Meat Production
2.1.1. Nitrogen Content (NC) per kg Meat
2.1.2. Nitrogen Loss (NL) per kg Meat Production
2.1.3. Total Nitrogen Intake (TNI) per kg Meat Production
2.1.4. Nitrogen Loss in Manure (NM) per kg Meat Production
2.1.5. Quantity of Manure (QM) to Be Treated for Nitrogen Recovery per kg Produced Meat
2.2. Ammonium Nitrogen Recovery (AR) from Manure per kg Produced Meat
2.3. Energy Demand (ED) for Manure Treatment
3. Results and Discussion
3.1. NL in Meat Production
3.2. Comparison among Countries
3.3. Energy Demand for Zero Liquid Discharge and Ammonium Nitrogen Recovery
4. Conclusions
- (i)
- This is the first study that formed a direct relationship between manure generation by beef, pork and poultry per unit of respective meat production. Nitrogen loss per unit of meat production was found to be directly proportional to the virtual nitrogen factors. The relationship between total nitrogen intake and the corresponding nitrogen loss per kg of meat production was also found linear;
- (ii)
- When comparing several countries, Japan was found to lose the highest amount of nitrogen for meat production, followed by Australia. Therefore, the amount of manure to be treated per unit of meat production was highest in Japan. The nitrogen loss due to meat production was found to be relatively lesser among the US and the European countries due to their advanced nitrogen recovery systems from waste streams;
- (iii)
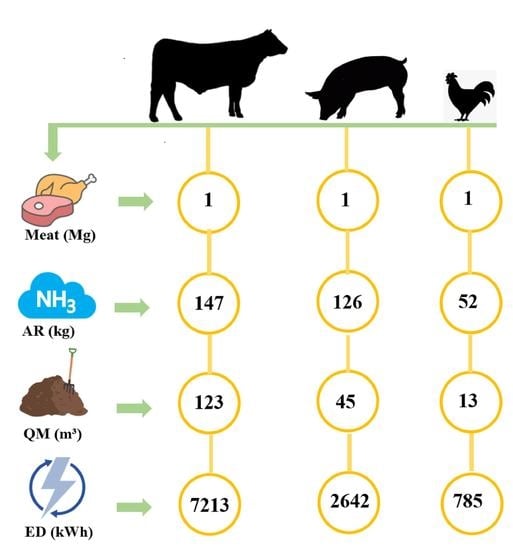
- The results showed that more than 7000 kWh energy was required to recover 140 kg of ammonium nitrogen from beef manure per 1 Mg meat production when considering the zero liquid discharge approach. The energy demand reduced significantly to below 3000 kWh and nearly 1000 kWh for pork and poultry manure treatment for the same.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Winiwarter, W.; Leip, A.; Leach, A.M.; Bleeker, A.; Erisman, J.W. Nitrogen footprints: Past, present and future. Environ. Res. Lett. 2014, 9, 115003. [Google Scholar] [CrossRef]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.R.; Leach, A.M.; de Vries, W. Consequences of human modification of the global nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef] [Green Version]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Karl, K.E.; Lancelot, C.; Gene, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 123, 1014–1015. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; van Grinsven, H.; Grizzetti, B. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Bodirsky, B.L.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Rolinski, S.; Weindl, I.; Schmitz, C.; Müller, C.; Bonsch, M.; Humpenöder, F. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 2014, 5, 3858. [Google Scholar] [CrossRef] [Green Version]
- Leach, A.M.; Galloway, J.N.; Bleeker, A.; Erisman, J.W.; Kohn, R.; Kitzes, J. A nitrogen footprint model to help consumers understand their role in nitrogen losses to the environment. Environ. Dev. 2012, 1, 40–66. [Google Scholar] [CrossRef] [Green Version]
- Oita, A.; Malik, A.; Kanemoto, K.; Geschke, A.; Nishijima, A.O.S.; Lenzen, M. Substantial nitrogen pollution embedded in international trade. Nat. Geosci. 2016, 9, 111–115. [Google Scholar] [CrossRef]
- Groenestein, C.; Hutchings, N.J.; Haenel, H.D.; Amon, B.; Menzi, H.; Mikkelsen, M.H.; Misselbrook, T.H.; van Bruggen, C.; Kupper, T.; Webb, J. Comparison of ammonia emissions related to nitrogen use efficiency of livestock production in Europe. J. Clean. Prod. 2019, 211, 1162–1170. [Google Scholar] [CrossRef]
- Stevens, C.J.; Leach, A.M.; Dale, S.; Galloway, J.N. Personal nitrogen footprint tool for the United Kingdom. Environ. Sci. Process. Impacts 2014, 16, 1563–1569. [Google Scholar] [CrossRef] [Green Version]
- Pierer, M.; Winiwarter, W.; Leach, A.M.; Galloway, J.N. The nitrogen footprint of food products and general consumption patterns in Austria. Food Policy 2014, 49, 128–136. [Google Scholar] [CrossRef]
- Liang, X.; Leach, A.; Galloway, J.N.; Gu, B.; Lam, S.K.; Chen, D. Beef and coal are key drivers of Australia’s high nitrogen footprint. Sci. Rep. 2016, 6, 39644. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Cattaneo, L.R.; Leach, A.; Galloway, J.N. First approach to the Japanese nitrogen footprint model to predict the loss of nitrogen to the environment. Environ. Res. Lett. 2014, 9, 115013. [Google Scholar] [CrossRef] [Green Version]
- Shibata, H.; Galloway, J.N.; Leach, A.M.; Cattaneo, L.R.; Noll, L.C.; Erisman, J.W.; Gu, B.; Liang, X.; Hayashi, K.; Ma, L.; et al. Nitrogen footprints: Regional realities and options to reduce nitrogen loss to the environment. Ambio 2016, 46, 129–142. [Google Scholar] [CrossRef]
- Hutton, M.O.; Leach, A.M.; Leip, A.; Galloway, J.N.; Bekunda, M.; Sullivan, C.; Lesschen, J.P. Toward a nitrogen footprint calculator for Tanzania. Environ. Res. Lett. 2017, 12, 034016. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.D.; Lassaletta, L. Nitrogen challenges in global livestock systems. Nat. Food 2020, 1, 400–401. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.; Abrol, Y.; Bekunda, M.; Datta, A.; Davidson, E.; de Vries, W.; Oenema, O. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution; Centre for Ecology & Hydrology: Edinburgh, UK, 2013. [Google Scholar]
- Bai, Z.; Ma, W.; Ma, L.; Velthof, G.L.; Wei, Z.; Havlík, P.; Oenema, O.; Lee, M.R.; Zhang, F. China’s livestock transition: Driving forces, impacts, and consequences. Sci. Adv. 2018, 4, eaar8534. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, R.; Zhang, A.; Yang, Z. Effect of Nitrate Leaching Caused by Swine Manure Application in Fields of the Yellow River Irrigation Zone of Ningxia, China. Sci. Rep. 2017, 7, 13693. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Shevliakova, E.; Stock, C.A.; Malyshev, S.; Milly, P.C.D. Prominence of the tropics in the recent rise of global nitrogen pollution. Nat. Commun. 2019, 10, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Li, W.; Li, X.; Ren, N. Spatial nitrifications of microbial processes during composting of swine, cow and chicken manure. Sci. Rep. 2015, 5, 14932. [Google Scholar] [CrossRef] [PubMed]
- Aneja, V.P.; Schlesinger, W.H.; Erisman, J.W. Farming pollution. Nat. Geosci. 2008, 1, 409–411. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Samanta, P.; Schwark, L.v.U.-S.; Horn, H.; Saravia, F. Nutrient recovery and ammonia-water production by MF-vacuum evaporation treatment of pig manure. J. Environ. Chem. Eng. 2021, 10, 106929. [Google Scholar] [CrossRef]
- Pikaar, I.; Matassa, S.; Rabaey, K.; Bodirsky, B.L.; Popp, A.; Herrero, M.; Verstraete, W. Microbes and the Next Nitrogen Revolution. Environ. Sci. Technol. 2017, 51, 7297–7303. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.; Wang, F.; Zhao, Q.; Zhao, T.; Xiang, J. Air stripping of ammonia in a water-sparged aerocyclone reactor. J. Hazard. Mater. 2009, 170, 983–988. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.; Christensen, M.; Sommer, S.G. Solid–Liquid Separation of Animal Slurry in Theory and Practice. In Sustainable Agriculture Volume 2; Springer: Berlin/Heidelberg, Germany, 2011; pp. 953–986. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.; Pellerin, Y. The use of membranes for the treatment of manure: A critical literature review. Biosyst. Eng. 2007, 98, 371–380. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass-Valorization 2016, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Biogas, F. Digestate as Fertilizer in Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. 2020. Available online: https://www.biogas.org/edcom/webfvb.nsf/id/BJHCPA-DE-Digestate-as-Fertilizer/$file/Digestate_as_Fertilizer.pdf (accessed on 31 January 2022).
- Liang, L.; Han, D. Energy-saving study of a system for ammonium sulfate recovery from wastewater with mechanical vapor compression (MVC). Res. J. Appl. Sci. Eng. Technol. 2011, 3, 1227–1232. [Google Scholar]
- Möller, K.; Schulz, R.; Müller, T. Substrate inputs, nutrient flows and nitrogen loss of two centralized biogas plants in southern Germany. Nutr. Cycl. Agroecosyst. 2010, 87, 307–325. [Google Scholar] [CrossRef]
- Gebhardt, S.; Lemar, L.; Haytowitz, D.; Pehrsson, P.; Nickle, M.; Showell, B.; Thomas, R.; Exler, J.; Holden, J. USDA National Nutrient Database for Standard Reference, Release 21; United States Department of AgricultureAgricultural Research Service: Washington, DC, USA, 2008.
- Guo, M.; Chen, X.; Bai, Z.; Jiang, R.; Galloway, J.N.; Leach, A.M.; Cattaneo, L.R.; Oenema, O.; Ma, L.; Zhang, F. How China’s nitrogen footprint of food has changed from 1961 to 2010. Environ. Res. Lett. 2017, 12, 104006. [Google Scholar] [CrossRef]
- Millet, S.; Aluwé, M.; van den Broeke, A.; Leen, F.; de Boever, J.; de Campeneere, S. Pork production with maximal nitrogen efficiency. Animal 2018, 12, 1060–1067. [Google Scholar] [CrossRef] [Green Version]
- Malomo, G.A.; Bolu, S.A.; Madugu, A.S.; Usman, Z.S. Nitrogen Emissions and Mitigation Strategies in Chicken Production. Anim. Husband. Nutr. 2018, 43, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.; Sørensen, P.; Velthof, G.; Amon, B.; Pinto, M.; Rodhe, L.; Salomon, E.; Hutchings, N.; Burczyk, P.; Reid, J. An Assessment of the Variation of Manure Nitrogen Efficiency throughout Europe and an Appraisal of Means to Increase Manure-N Efficiency. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2013; Volume 119, pp. 371–442. [Google Scholar]
- Sánchez-Hernández, E.; Weiland, P.; Borja, R. The effect of biogas sparging on cow manure characteristics and its subsequent anaerobic biodegradation. Int. Biodeterior. Biodegrad. 2013, 83, 10–16. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.; Frost, J.; Hu, Z.; Zhan, X. Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour. Technol. 2011, 102, 5728–5733. [Google Scholar] [CrossRef]
- Yangin-Gomec, C.; Ozturk, I. Effect of maize silage addition on biomethane recovery from mesophilic co-digestion of chicken and cattle manure to suppress ammonia inhibition. Energy Convers. Manag. 2013, 71, 92–100. [Google Scholar] [CrossRef]
- Matassa, S.; Batstone, D.J.; Hülsen, T.; Schnoor, J.; Verstraete, W. Can Direct Conversion of Used Nitrogen to New Feed and Protein Help Feed the World? Environ. Sci. Technol. 2015, 49, 5247–5254. [Google Scholar] [CrossRef]
- Eshel, G.; Shepon, A.; Makov, T.; Milo, R. Land, irrigation water, greenhouse gas, and reactive nitrogen burdens of meat, eggs, and dairy production in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 11996–12001. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, A.; Else, P. Basal metabolic rate: History, composition, regulation, and usefulness. Physiol. Biochem. Zool. 2004, 77, 869–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leip, A.; Weiss, F.; Lesschen, J.P.; Westhoek, H. The nitrogen footprint of food products in the European Union. J. Agric. Sci. 2013, 152, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Ti, C.; Vitousek, P.M.; Chen, D.; Leip, A.; Cai, Z.; Zhu, Z. Fertilizer nitrogen recovery efficiencies in crop production systems of China with and without consideration of the residual effect of nitrogen. Environ. Res. Lett. 2014, 9, 095002. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Velthof, G.; Wang, F.; Qin, W.; Zhang, W.; Liu, Z.; Zhang, Y.; Wei, J.; Lesschen, J.; Ma, W.; et al. Nitrogen and phosphorus use efficiencies and losses in the food chain in China at regional scales in 1980 and 2005. Sci. Total Environ. 2012, 434, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldt, H.C.; Hall, N.G. Dietary protein quality and malnutrition in Africa. Br. J. Nutr. 2012, 108, S69–S76. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samanta, P.; Horn, H.; Saravia, F. Impact of Livestock Farming on Nitrogen Pollution and the Corresponding Energy Demand for Zero Liquid Discharge. Water 2022, 14, 1278. https://doi.org/10.3390/w14081278
Samanta P, Horn H, Saravia F. Impact of Livestock Farming on Nitrogen Pollution and the Corresponding Energy Demand for Zero Liquid Discharge. Water. 2022; 14(8):1278. https://doi.org/10.3390/w14081278
Chicago/Turabian StyleSamanta, Prantik, Harald Horn, and Florencia Saravia. 2022. "Impact of Livestock Farming on Nitrogen Pollution and the Corresponding Energy Demand for Zero Liquid Discharge" Water 14, no. 8: 1278. https://doi.org/10.3390/w14081278
APA StyleSamanta, P., Horn, H., & Saravia, F. (2022). Impact of Livestock Farming on Nitrogen Pollution and the Corresponding Energy Demand for Zero Liquid Discharge. Water, 14(8), 1278. https://doi.org/10.3390/w14081278