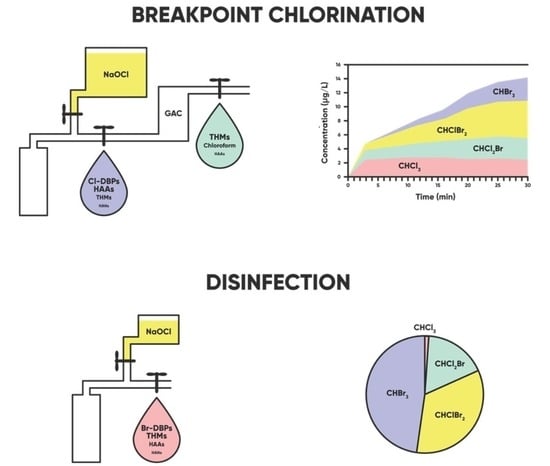
Comparison of Disinfection By-Product Formation and Distribution during Breakpoint Chlorination and Chlorine-Based Disinfection in Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Bench-Scale Experiments
2.2.1. Raw Water Composition
2.2.2. Breakpoint Chlorination
2.2.3. Disinfection
2.3. Analytical Methods
2.4. Data Analysis
3. Results and Discussion
3.1. Field Sampling
3.1.1. Raw Water and Technology Parameters
3.1.2. DBP Formation
Organic DBPs
Chlorate
3.1.3. Bromine Substitution Factors
3.1.4. Efficiency of GAC Filtration
3.2. Bench-Scale Experiments
3.2.1. Breakpoint Chlorination
Bromide to Chlorine Ratio
Contact Time
3.2.2. Disinfection
Bromide to Chlorine Ratio
3.2.3. Comparison of Breakpoint Chlorination and Disinfection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellar, T.A.; Lichtenberg, J.J.; Kroner, R.C. Occurrence of Organohalides in Chlorinated Drinking Waters. J. Am. Water Work. Assoc. 1974, 66, 703–706. [Google Scholar] [CrossRef]
- Miller, J.W.; Uden, P.C. Characterization of nonvolatile aqueous chlorination products of humic substances. Environ. Sci. Technol. 1983, 17, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Shi, Q.; Hu, J.; Chu, M.; Yu, J.; Yang, M. Study on Transformation of Natural Organic Matter in Source Water during Chlorination and Its Chlorinated Products using Ultrahigh Resolution Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 4396–4402. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, H.S.; Krasner, S.W.; Richardson, S.D.; Thruston, A.D. The Occurrence of Disinfection By-Products (DBPs) of Health Concern in Drinking Water: Results of a Nationwide DBP Occurrence Study; National Exposure Research Laboratory, U.S. Environmental Protection Agency: Athens, GA, USA, 2002.
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Krasner, S.W.; Kostopoulou, M.; Toledano, M.B.; Wright, J.; Patelarou, E.; Kogevinas, M.; Villanueva, C.M.; Carrasco-Turigas, G.; Marina, L.S.; Fernández-Somoano, A.; et al. Occurrence of DBPs in Drinking Water of European Regions for Epidemiology Studies. J. AWWA 2016, 108, E501–E512. [Google Scholar] [CrossRef]
- De Castro Medeiros, L.; de Alencar, F.L.S.; Navoni, J.A.; de Araujo, A.L.C.; do Amaral, V.S. Toxicological aspects of trihalomethanes: A systematic review. Environ. Sci. Pollut. Res. 2019, 26, 5316–5332. [Google Scholar] [CrossRef]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 11 April 2022).
- IRIS Toxicological Review of Dichloroacetic Acid (CAS No. 79-43-6); U.S. Environmental Protection Agency: Washington, DC, USA, 2003.
- IRIS Toxicological Review of Trichloroacetic Acid (CAS No. 76-03-9); U.S. Environmental Protection Agency: Washington, DC, USA, 2011.
- US EPA: Ground Water and Drinking Water: National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 11 April 2022).
- The European Parliament and the Council of the European Union Directive (EU) 2020/2184, EU (revised) Drinking Water Directive. Annex 1. Part B. Off. J. Eur. Communities 2020, 2019, 35.
- Muellner, M.G.; Wagner, E.D.; Mccalla, K.; Richardson, S.D.; Woo, Y.T.; Plewa, M.J. Haloacetonitriles vs. regulated haloacetic acids: Are nitrogen-containing DBFs more toxic? Environ. Sci. Technol. 2007, 41, 645–651. [Google Scholar] [CrossRef]
- Plewa, M.J.; Wagner, E.D.; Muellner, M.G.; Hsu, K.-M.; Richardson, S.D. Comparative Mammalian Cell Toxicity of N-DBPs and C-DBPs. In Disinfection By-Products in Drinking Water; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 995, pp. 3–36. ISBN 9780841269507. [Google Scholar]
- Bond, T.; Templeton, M.R.; Graham, N. Precursors of nitrogenous disinfection by-products in drinking water—A critical review and analysis. J. Hazard. Mater. 2012, 235–236, 1–16. [Google Scholar] [CrossRef]
- Zeng, T.; Plewa, M.J.; Mitch, W.A. N-Nitrosamines and halogenated disinfection byproducts in U.S. Full Advanced Treatment trains for potable reuse. Water Res. 2016, 101, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Bufa-Dőrr, Z.; Málnási, T.; Oravecz, O.; Vargha, M.; Vecsey, A. Magyarország ivóvízminősége 2019 (Qualitiy of drinking water in Hungary in year 2019); National Public Health Center: Budapest, Hungary, 2021.
- Fossen Johnson, S. Methemoglobinemia: Infants at risk. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.A.; Kimura, S.Y.; Liberatore, H.K.; Summers, R.S.; Knappe, D.R.U.; Stanford, B.D.; Maness, J.C.; Mulhern, R.E.; Selbes, M.; Richardson, S.D. Does Granular Activated Carbon with Chlorination Produce Safer Drinking Water? from Disinfection Byproducts and Total Organic Halogen to Calculated Toxicity. Environ. Sci. Technol. 2019, 53, 5987–5999. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.A.; Kimura, S.Y.; Liberatore, H.K.; Knappe, D.R.U.; Stanford, B.; Summers, R.S.; Dickenson, E.R.; Maness, J.C.; Glover, C.; Selbes, M.; et al. GAC to BAC: Does it make chloraminated drinking water safer? Water Res. 2020, 172, 115432. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zainudin, F.; Abu Hasan, H.; Sheikh Abdullah, S.R. An overview of the technology used to remove trihalomethane (THM), trihalomethane precursors, and trihalomethane formation potential (THMFP) from water and wastewater. J. Ind. Eng. Chem. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.M.; Ahn, H.T.; Chung, M.; Le, X.A.; Saini, D.; Bhati, A.; Sonkar, S.K.; Kim, M.I.; Kim, T. N, S, and P-co-Doped Carbon Quantum Dots: Intrinsic Peroxidase Activity in a Wide pH Range and Its Antibacterial Applications. ACS Biomater. Sci. Eng. 2020, 6, 5527–5537. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; González, E.P.V.; Lorza, R.L.; Gómez, F.S. Effecting partial elimination of isocyanuric acid from swimming pool water systems. Water 2019, 11, 712. [Google Scholar] [CrossRef] [Green Version]
- Bond, T.; Huang, J.; Graham, N.J.D.; Templeton, M.R. Examining the interrelationship between DOC, bromide and chlorine dose on DBP formation in drinking water—A case study. Sci. Total Environ. 2014, 470–471, 469–479. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. DBP formation during chlorination and chloramination: Effect of reaction time. pH, dosage, and temperature. J. Am. Water Work. Assoc. 2008, 100, 82–95. [Google Scholar] [CrossRef]
- Hong, H.; Xiong, Y.; Ruan, M.; Liao, F.; Lin, H.; Liang, Y. Factors affecting THMs, HAAs and HNMs formation of Jin Lan Reservoir water exposed to chlorine and monochloramine. Sci. Total Environ. 2013, 444, 196–204. [Google Scholar] [CrossRef]
- Garcia-Villanova, R.J.; Oliveira Dantas Leite, M.V.; Hernández Hierro, J.M.; de Castro Alfageme, S.; García Hernández, C. Occurrence of bromate, chlorite and chlorate in drinking waters disinfected with hypochlorite reagents. Tracing their origins. Sci. Total Environ. 2010, 408, 2616–2620. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization ISO 7393-1:1985 Water quality—Determination of free chlorine and total chlorine—Part 1: Titrimetric method using N,N-diethyl-1,4-phenylenediamine. Available online: https://www.iso.org/obp/ui/#iso:std:iso:7393:-1:ed-1:v1:en (accessed on 17 March 2022).
- EN 1484:1998; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). European Committee for Standardization: Brussels, Belgium, 1997.
- ISO 7150-1:1992; Water Quality. Determination of Ammonium. Part 1: Manual Spectrophotometric Method. International Organization for Standardization: Geneva, Switzerland, 1992.
- International Organization for Standardization ISO 10304-1:2007 Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10304:-1:ed-2:v1:en (accessed on 17 March 2022).
- METHOD 552. 3 Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid-Liquid Microextraction, Derivatization, and Gas Chromatography with Electron Capture Detection; Office of Ground Water and Drinking Water: Cincinnati, OH, USA, 2003; pp. 1–55.
- METHOD 551. 1 Determination of Chlorination Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography with Electron-Capture Detection; Office of Ground Water and Drinking Water: Cincinnati, OH, USA, 1990; pp. 1–61.
- ISO 10304-4:2000; Water Quality—Determination of Dissolved Anions by Liquid Chromatography of ions—Part 4: Determination of Chlorate, Chloride and Chlorite in Water with Low Contamination. International Organization for Standardization: Geneva, Switzerland, 2000.
- Obolensky, A.; Singer, P.C. Halogen substitution patterns among disinfection byproducts in the information collection rule database. Environ. Sci. Technol. 2005, 39, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Margerum, D.W. Kinetics and Mechanism of General-Acid-Assisted Oxidation of Bromide by Hypochlorite and Hypochlorous Acid. Inorg. Chem. 1987, 26, 2706–2711. [Google Scholar] [CrossRef]
- Haas, C.N. Disinfection. In Water Qualitiy and Treatment: A Handbook of Community Water Supplies; Letterman, R.D., Ed.; McGraw-Hill Inc.: New York, NY, USA, 1999; pp. 14.1–14.60. ISBN 0070016593. [Google Scholar]
- Uyak, V.; Toroz, I. Investigation of bromide ion effects on disinfection by-products formation and speciation in an Istanbul water supply. J. Hazard. Mater. 2007, 149, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. Evaluation of bromine substitution factors of DBPs during chlorination and chloramination. Water Res. 2012, 46, 4208–4216. [Google Scholar] [CrossRef] [PubMed]
- Snoeyink, V.L.; Summers, R.S. Adsorption of organic compounds. In Water Quality and Treatment: A Handbook of Community Water Supplies; Letterman, R.D., Ed.; McGraw-Hill Inc.: New York, NY, USA, 1999; pp. 13.1–13.83. ISBN 0070016593. [Google Scholar]
- Babi, K.G.; Koumenides, K.M.; Nikolaou, A.D.; Makri, C.A.; Tzoumerkas, F.K.; Lekkas, T.D. Pilot study of the removal of THMs, HAAs and DOC from drinking water by GAC adsorption. Desalination 2007, 210, 215–224. [Google Scholar] [CrossRef]
- Kim, J.; Kang, B. DBPs removal in GAC filter-adsorber. Water Res. 2008, 42, 145–152. [Google Scholar] [CrossRef]
- Tang, H.L.; Xie, Y.F. Biologically active carbon filtration for haloacetic acid removal from swimming pool water. Sci. Total Environ. 2016, 541, 58–64. [Google Scholar] [CrossRef]
- Wu, H.; Xie, Y.F. Effects of EBCT and water temperature on HAA removal using BAC. J. Am. Water Work. Assoc. 2005, 97, 94–101. [Google Scholar] [CrossRef]
- Hoslett, J.; Massara, T.M.; Malamis, S.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Surface water filtration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Extending granular activated carbon (GAC) bed life: A column study of in-situ chemical regeneration of pesticide loaded activated carbon for water treatment. Chemosphere 2022, 286, 131888. [Google Scholar] [CrossRef] [PubMed]
- Ek, M.; Baresel, C.; Magnér, J.; Bergström, R.; Harding, M. Activated carbon for the removal of pharmaceutical residues from treated wastewater. Water Sci. Technol. 2014, 69, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuDalo, M.A.; Nevostrueva, S.; Hernandez, M.T. Enhanced Copper (II) Removal from Acidic Water By Granular Activated Carbon Impregnated with Carboxybenzotriazole. Apcbee Procedia 2013, 5, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhang, J.; Kang, Y.; Liu, H. Rapid and efficient removal of Pb(II) from aqueous solutions using biomass-derived activated carbon with humic acid in-situ modification. Ecotoxicol. Environ. Saf. 2017, 145, 442–448. [Google Scholar] [CrossRef]
- Liu, C.; Olivares, C.I.; Pinto, A.J.; Lauderdale, C.V.; Brown, J.; Selbes, M.; Karanfil, T. The control of disinfection byproducts and their precursors in biologically active filtration processes. Water Res. 2017, 124, 630–653. [Google Scholar] [CrossRef]
- Jafvert, C.T.; Valentine, R.L. Reaction Scheme for the Chlorination of Ammoniacal Water. Environ. Sci. Technol. 1992, 26, 577–586. [Google Scholar] [CrossRef]
- Liang, L.; Singer, P.C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ. Sci. Technol. 2003, 37, 2920–2928. [Google Scholar] [CrossRef]
- Zhang, X.; Minear, R.A. Decomposition of trihaloacetic acids and formation of the corresponding trihalomethanes in drinking water. Water Res. 2002, 36, 3665–3673. [Google Scholar] [CrossRef]
- Stefán, D.; Erdélyi, N.; Izsák, B.; Záray, G.; Vargha, M. Formation of chlorination by-products in drinking water treatment plants using breakpoint chlorination. Microchem. J. 2019, 149, 104008. [Google Scholar] [CrossRef]
- Ledesma, B.; Román, S.; Álvarez-Murillo, A.; Sabio, E.; González, J.F. Cyclic adsorption/thermal regeneration of activated carbons. J. Anal. Appl. Pyrolysis 2014, 106, 112–117. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Insights into chemical regeneration of activated carbon for water treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Chemical regeneration of granular activated carbon: Preliminary evaluation of alternative regenerant solutions. Environ. Sci. Water Res. Technol. 2020, 6, 2043–2056. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Kalankesh, L.R. Removal of precursors and disinfection byproducts (DBPs) by membrane filtration from water; a review. J. Environ. Health Sci. Eng. 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.; Lei, Y.; Zhang, X.; Yang, X. Treating disinfection byproducts with UV or solar irradiation and in UV advanced oxidation processes: A review. J. Hazard. Mater. 2021, 408, 124435. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Measurement Principle | Measurement Methods | Limit of Quantitation (LOQ) | |
|---|---|---|---|---|
| On site | temperature, pH, conductivity | - | - | - |
| free and combined chlorine | DPD colorimetric titration | ISO 7393-1:1985 [29] | 0.030 mg Cl2/L | |
| Basic parameters | DOC | combustion + IR detection | UNE EN 1484:1998 [30] | 0.50 mg/L |
| ammonium | photometric | ISO 7150-1:1992 [31] | 0.020 mg/L | |
| chloride, bromide, nitrite, nitrate | IC + conductivity detection | ISO 10304-1:2007 [32] | 2.0, 0.050, 0.030, and 0.50 mg/L, respectively | |
| Organic DBPs | 4 THMs: chloroform, bromo-dichloro-methane (BDCM), dibromo-chloromethane (DBCM), bromoform | Purge & Trap-GC-MS | - | 0.10 µg/L |
| 9 HAAs: monochloroacetic acid (MCAA), monobromoacetic acid (MBAA), dichloroacetic acid (DCAA), trichloroacetic acid (TCAA), bromo-chloroacetic acid (BCAA), dibromoacetic acid (DBAA), bromo-dichloroacetic acid (BDCAA), dibromo-chloroacetic acid (DBCAA), tribromoacetic acid (TBAA) | Liquid-liquid extraction + derivatization + GC-MS | EPA 552.3 [33] with slight changes | 0.50 µg/L (except MCAA: 1.0 µg/L) | |
| 3 HANs: dichloroacetonitrile (DCAN), bromo-chloroacetonitrile (BCAN), dibromoacetonitrile (DBAN) | Liquid-liquid extraction + GC-ECD | EPA 551.1 [34] with minor modification | 0.30 µg/L | |
| Inorganic DBPs | Chlorate | IC + cond. detection | ISO 10304-4:2000 [35] | 0.050 mg/L |
| Parameters | Waterwork I | Waterwork II | Waterwork III | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Raw water parameters (n = 8) | Temperature (°C) | 17.0 | 0.1 | 18.8 | 0.7 | 15.2 | 0.5 |
| pH | 7.97 | 0.08 | 7.67 | 0.10 | 7.75 | 0.08 | |
| Cond (µS/cm) | 812 | 7 | 711 | 10 | 643 | 40 | |
| NH3-N (mg/L) | 1.2 | 0.04 | 1.0 | 0.03 | 0.84 | 0.11 | |
| Br− (mg/L) | 0.17 | 0.02 | <0.05 | - | <0.05 | - | |
| DOC (mg/L) | 1.0 | 0.2 | 2.3 | 0.2 | 2.2 | 0.2 | |
| Technology parameters (n = 8) | Res. free chlorine at breakpoint (mg Cl2/L) | 3.0 | 0.91 | 2.9 | 0.95 | 2.9 | 0.88 |
| Res. combined chlorine at disinfection (mg Cl2/L) | 0.60 | 0.20 | 0.58 | 0.11 | 0.70 | 0.24 | |
| DBPs | Waterwork I | Waterwork II | Waterwork III | |||
|---|---|---|---|---|---|---|
| BC | Disinf. | BC | Disinf. | BC | Disinf. | |
| THMs | 0.14 (0.024) | 0.72 (0.025) | 0.021 (0.006) | 0.15 (0.034) | 0.011 (0.006) | 0.13 (0.11) |
| DHAAs | 0.35 (0.15) | 0.71 (0.11) | 0.044 (0.030) | 0.35 (0.19) | 0.021 (0.018) | 0.14 (0.17) |
| THAAs | 0.093 (0.042) | 0.39 (0.17) | 0.027 (0.013) | 0.079 (0.014) | 0.011 (0.009) | 0.049 (0.037) |
| DHANs | 0.33 (0.040) | 0.88 (0.023) | 0.056 (0.013) | 0.14 (0.014) | 0.024 (0.020) | 0.057 (0.040) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefán, D.; Balogh, J.; Záray, G.; Vargha, M. Comparison of Disinfection By-Product Formation and Distribution during Breakpoint Chlorination and Chlorine-Based Disinfection in Drinking Water. Water 2022, 14, 1372. https://doi.org/10.3390/w14091372
Stefán D, Balogh J, Záray G, Vargha M. Comparison of Disinfection By-Product Formation and Distribution during Breakpoint Chlorination and Chlorine-Based Disinfection in Drinking Water. Water. 2022; 14(9):1372. https://doi.org/10.3390/w14091372
Chicago/Turabian StyleStefán, Dávid, Judit Balogh, Gyula Záray, and Márta Vargha. 2022. "Comparison of Disinfection By-Product Formation and Distribution during Breakpoint Chlorination and Chlorine-Based Disinfection in Drinking Water" Water 14, no. 9: 1372. https://doi.org/10.3390/w14091372
APA StyleStefán, D., Balogh, J., Záray, G., & Vargha, M. (2022). Comparison of Disinfection By-Product Formation and Distribution during Breakpoint Chlorination and Chlorine-Based Disinfection in Drinking Water. Water, 14(9), 1372. https://doi.org/10.3390/w14091372