Salt Removal by Chemically Modified Graphene in Capacitive Deionization (CDI)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
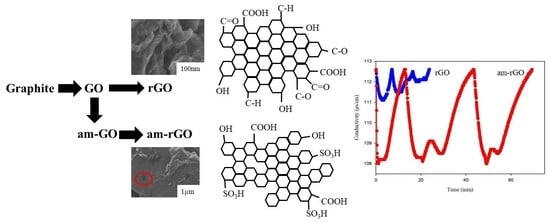
3.1. Physical and Chemical Characterization
3.2. Electrochemical Properties
3.3. CDI Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sher, F.; Iqbal, S.Z.; Rasheed, T.; Hanif, K.; Sulejmanović, J.; Zafar, F.; Lima, E.C. Coupling of electrocoagulation and powder activated carbon for the treatment of sustainable wastewater. Environ. Sci. Pollut. Res. 2021, 28, 48505–48516. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Sher, F.; Iqbal, H.M.N. Tailored functional materials as robust candidates to mitigate pesticides in aqueous matrices—A review. Chemosphere 2021, 282, 131056. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Kausar, F.; Rizwan, K.; Adeel, M.; Sher, F.; Alwadai, N.; Alshammari, F.H. Two dimensional MXenes as emerging paradigm for adsorptive removal of toxic metallic pollutants from wastewater. Chemosphere 2022, 287, 132319. [Google Scholar] [CrossRef] [PubMed]
- Sulejmanović, J.; Kovač, N.; Memić, M.; Šabanović, E.; Begić, S.; Sher, F. Selective removal of lead ions from aqueous solutions using SiO2–MoO3: Isotherm, kinetics and thermodynamic studies. Case Stud. Chem. Environ. Eng. 2021, 3, 100083. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; Ablouh, E.-H.; Bouich, A.; Lacherai, A.; Jada, A.; Lima, E.C.; Sher, F. Biosynthesis of SiO2 nanoparticles using extract of Nerium oleander leaves for the removal of tetracycline antibiotic. Chemosphere 2022, 287, 132453. [Google Scholar] [CrossRef]
- Américo-Pinheiro, J.H.P.; Paschoa, C.V.M.; Salomão, G.R.; Cruz, I.A.; Isique, W.D.; Ferreira, L.F.R.; Sher, F.; Torres, N.H.; Kumar, V.; Pinheiro, R.S.B. Adsorptive remediation of naproxen from water using in-house developed hybrid material functionalized with iron oxide. Chemosphere 2022, 289, 133222. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of capacitive deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Myint, M.T.Z.; Al-Harthi, S.H.; Dutta, J. Brackish water desalination by capacitive deionization using zinc oxide micro/nanostructures grafted on activated carbon cloth electrodes. Desalination 2014, 344, 236–242. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Bao, S.; Cruz, R.; Song, S. Desalination by capacitive deionization process using nitric acid-modified activated carbon as the electrodes. Desalination 2014, 340, 67–72. [Google Scholar] [CrossRef]
- Seo, S.-J.; Jeon, H.; Lee, J.K.; Kim, G.-Y.; Park, D.; Nojima, H.; Lee, J.; Moon, S.-H. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, F.; Lan, H.; Liu, H.; Qu, J. Preparation of a manganese dioxide/carbon fiber electrode for electrosorptive removal of copper ions from water. J. Colloid Interface Sci. 2015, 446, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, J.-H. Enhanced desalination efficiency in capacitive deionization with an ion-selective membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; van der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes. J. Electrochem. Soc. 1996, 143, 159–169. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, D.; Shi, L.; Yan, T. High performance ordered mesoporous carbon/carbon nanotube composite electrodes for capacitive deionization. J. Mater. Chem. 2012, 22, 6603–6612. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Shi, L.; Peng, Z.; Wen, X.; Zhang, J. Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J. Mater. Chem. 2012, 22, 14696–14704. [Google Scholar] [CrossRef]
- Jia, B.; Zou, L. Wettability and its influence on graphene nansoheets as electrode material for capacitive deionization. Chem. Phys. Lett. 2012, 548, 23–28. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Pan, L.; Sun, Z. Novel graphene-like electrodes for capacitive deionization. Environ. Sci. Technol. 2010, 44, 8692–8697. [Google Scholar] [CrossRef]
- Li, H.; Lu, T.; Pan, L.; Zhang, Y.; Sun, Z. Electrosorption behavior of graphene in NaCl solutions. J. Mater. Chem. 2009, 19, 6773–6779. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sakthivel, T.; Gunasekaran, V.; Kim, S.-J. Effect of oxygenated functional groups on the photoluminescence properties of graphene-oxide nanosheets. Mater. Sci. Semicond. Process. 2014, 19, 174–178. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Fan, C.-S.; Hou, C.-H. Electro-enhanced removal of copper ions from aqueous solutions by capacitive deionization. J. Hazard. Mater. 2014, 278, 8–15. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, F.; Liu, K.; Deng, H.; Zhang, Q.; Feng, J.; Fu, Q. A simple and efficient method to prepare graphene by reduction of graphite oxide with sodium hydrosulfite. Nanotechnology 2010, 22, 045704. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Lima, E.C.; Benetti, A.D.; Thue, P.S.; Cunha, M.R.; Cimirro, N.F.; Sher, F.; Dehghani, M.H.; dos Reis, G.S.; Dotto, G.L. Preparation of hybrids of wood sawdust with 3-aminopropyl-triethoxysilane. Application as an adsorbent to remove Reactive Blue 4 dye from wastewater effluents. J. Taiwan Inst. Chem. Eng. 2021, 125, 141–152. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Chen, Z.; Zhang, H.; Wu, C.; Wang, Y.; Li, W. A study of electrosorption selectivity of anions by activated carbon electrodes in capacitive deionization. Desalination 2015, 369, 46–50. [Google Scholar] [CrossRef]
- El Deen, A.G.; Barakat, N.; Khalil, K.A.; Motlak, M.; Kim, H.Y. Graphene/SnO2 nanocomposite as an effective electrode material for saline water desalination using capacitive deionization. Ceram. Int. 2014, 40, 14627–14634. [Google Scholar] [CrossRef]
- Xu, X.; Pan, L.; Liu, Y.; Lu, T.; Sun, Z. Enhanced capacitive deionization performance of graphene by nitrogen doping. J. Colloid Interface Sci. 2015, 445, 143–150. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]








| Sample | Pore Volume (Vtot)(cm3/g) | Pore Size (nm) | BET Surface Area (m2/g) | Vmicro a (cm3/g) | Vmeso b (cm3/g) | Vmeso/Vtot (%) |
|---|---|---|---|---|---|---|
| rGO | 0.0219 | 6.97 | 12.56 | 0.0009 | 0.021 | 95.9 |
| am-rGO | 0.0217 | 14.46 | 6.00 | 0.0017 | 0.020 | 92.2 |
| CDI Cycle | rGO | am-rGO | ||||
|---|---|---|---|---|---|---|
| Removal Efficiency (%) | Electrosorption Capacity | Removal Efficiency (%) | Electrosorption Capacity | |||
| mg NaCl/g | μmol NaCl/g | mg NaCl/g | μmol NaCl/g | |||
| 1 | 1.3 | 0.23 | 3.97 | 3.5 | 0.63 | 10.86 |
| 2 | 1.0 | 0.18 | 3.10 | 3.5 | 0.63 | 10.86 |
| 3 | 0.4 | 0.07 | 1.21 | 3.5 | 0.63 | 10.86 |
| Average | 0.85 | 0.16 | 2.76 | 3.5 | 0.63 | 10.86 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Reducing Agent | [NaCl] (mg/L) | Applied Voltage (V) | Electrosorption Capacity (μmol NaCl/g) | Ref |
|---|---|---|---|---|---|---|---|---|
| PG 1 | 154.7 | 0.87 | - | N2(g) annealing | 200 | 1.8 | 65.8 | [30] |
| NG 2 | 358.9 | 1.02 | - | NH3(g) annealing | 200 | 1.8 | 82.2 | [30] |
| GNC 3 | 299 | - | 3.6 | Fe powder | 250 | 2.0 | 70.1 | [18] |
| S-GNC 4 | 464 | - | 3.3 | Fe powder | 250 | 2.0 | 147.0 | [18] |
| rGO | 14.2 | - | 7.42 | hydrazine | 96 | 2.0 | 31.6 | [21] |
| GNF 5 | 222.01 | 51.01 | - | hydrazine | 25 | 2.0 | 23.2 | [20] |
| 25 | 1.0 | 6.0 | [20] | |||||
| rGO | 12.56 | 0.0219 | 6.97 | HMTA | 50 | 1.0 | 2.76 | This work |
| am-rGO | 6.00 | 0.0217 | 14.46 | HMTA | 50 | 1.0 | 10.86 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.-Y.; Chen, Y.-F.; Wang, C.-Y. Salt Removal by Chemically Modified Graphene in Capacitive Deionization (CDI). Water 2022, 14, 1379. https://doi.org/10.3390/w14091379
Peng C-Y, Chen Y-F, Wang C-Y. Salt Removal by Chemically Modified Graphene in Capacitive Deionization (CDI). Water. 2022; 14(9):1379. https://doi.org/10.3390/w14091379
Chicago/Turabian StylePeng, Ching-Yu, Yi-Fang Chen, and Ching-Yi Wang. 2022. "Salt Removal by Chemically Modified Graphene in Capacitive Deionization (CDI)" Water 14, no. 9: 1379. https://doi.org/10.3390/w14091379
APA StylePeng, C. -Y., Chen, Y. -F., & Wang, C. -Y. (2022). Salt Removal by Chemically Modified Graphene in Capacitive Deionization (CDI). Water, 14(9), 1379. https://doi.org/10.3390/w14091379