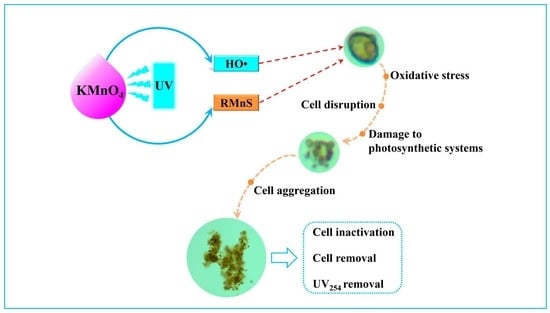
Efficient Inactivation and Removal of a Harmful Marine Algae—Heterosigma akashiwo—By UV-Assisted Permanganate Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Reactor and Procedures
2.3. Cell Removal
2.4. Analytical Methods
3. Results
3.1. Algal Cell Morphology and Removal during UV/KMnO4 Treatment
3.2. KMnO4 Decay
3.3. The Change in the UV254 and Hemolysis Rate
3.4. Content of Photosynthetic Pigments and Antioxidant System Activity
3.5. Removal Efficiency of Algal Cells via a Settling Test
4. Discussion
4.1. The Inactivation of H. akashiwo Cells by UV/KMnO4
4.2. The Impacts of UV/KMnO4 Treatment on Water Quality
4.3. Algal Cell Removal during the UV/KMnO4 and Subsequent Self-Settling Processes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Basti, L.; Go, J.; Okano, S.; Higuchi, K.; Nagai, S.; Nagai, K. Sublethal and antioxidant effects of six ichthyotoxic algae on early-life stages of the Japanese pearl oyster. Harmful Algae 2021, 103, 102013. [Google Scholar] [CrossRef]
- Sandoval-Sanhueza, A.; Aguilera-Belmonte, A.; Basti, L.; Figueroa, R.I.; Molinet, C.; Alvarez, G.; Oyanedel, S.; Riobo, P.; Mancilla-Gutierrez, G.; Diaz, P.A. Interactive effects of temperature and salinity on the growth and cytotoxicity of the fish-killing microalgal species Heterosigma akashiwo and Pseudochattonella verruculosa. Mar. Pollut. Bull. 2022, 174, 113234. [Google Scholar] [CrossRef]
- Butrón, A.; Madariaga, I.; Orive, E. Tolerance to high irradiance levels as a determinant of the bloom-forming Heterosigma akashiwo success in estuarine waters in summer. Estuar. Coast. Shelf Sci. 2012, 107, 141–149. [Google Scholar] [CrossRef]
- Butrón, A.; Orive, E.; Madariaga, I. Potential risk of harmful algae transport by ballast waters: The case of Bilbao Harbour. Mar. Pollut. Bull. 2011, 62, 747–757. [Google Scholar] [CrossRef]
- Tobin, E.D.; Grunbaum, D.; Patterson, J.; Cattolico, R.A. Behavioral and physiological changes during benthic-pelagic transition in the harmful alga, Heterosigma akashiwo: Potential for rapid bloom formation. PLoS ONE 2013, 8, e76663. [Google Scholar] [CrossRef]
- Caron, D.A.; Garneau, M.-È.; Seubert, E.; Howard, M.D.A.; Darjany, L.; Schnetzer, A.; Cetinić, I.; Filteau, G.; Lauri, P.; Jones, B.; et al. Harmful algae and their potential impacts on desalination operations off southern California. Water Res. 2010, 44, 385–416. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Tabatabai, S.A.A.; Dhakal, N.; Amy, G.; Schippers, J.C.; Kennedy, M.D. Algal blooms: An emerging threat to seawater reverse osmosis desalination. Desalin. Water Treat. 2014, 55, 2601–2611. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Haarhoff, J. Seawater pretreatment for reverse osmosis: Chemistry, contaminants, and coagulation. Water Res. 2011, 45, 5428–5440. [Google Scholar] [CrossRef]
- Romero-Martinez, L.; Rivas-Zaballos, I.; Moreno-Andres, J.; Moreno-Garrido, I.; Acevedo-Merino, A.; Nebot, E. Effect of the length of dark storage following ultraviolet irradiation of Tetraselmis suecica and its implications for ballast water management. Sci. Total Environ. 2020, 711, 134611. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Gao, N.; Deng, Y.; Qiao, J.; Zhang, K.; Li, T.; Dong, L. Mechanistic studies of Microcystic aeruginosa inactivation and degradation by UV-C irradiation and chlorination with poly-synchronous analyses. Desalination 2011, 272, 107–119. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, F.; Xu, B.; Ma, G.; Zhang, L.; Yang, Z.; Liu, R.; Sun, C.; Cheng, X.; Guo, N.; et al. Iron-based technology coupling moderate preoxidation with hybrid coagulation for highly effective removal and moderate growth inhibition of Oscillatoria in drinking water treatment plants. J. Environ. Chem. Eng. 2022, 10, 107723. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Cao, X.; Song, X. Fractal dimensions of flocs between clay particles and HAB organisms. Chin. J. Oceanol. Limnol. 2011, 29, 656–663. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, X.; Liu, X.; Ge, F.; Cheng, P. Coagulation performance and floc properties of Microcystis aeruginosa in the presence of humic acid. Water Supply 2015, 15, 339–347. [Google Scholar] [CrossRef]
- Piezer, K.; Li, L.; Jeon, Y.; Kadudula, A.; Seo, Y. The application of potassium permanganate to treat cyanobacteria-laden water: A Review. Process Saf. Environ. 2021, 148, 400–414. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, J.; Li, A.; Xie, R.; Liang, Z.; Wang, A.; Ling, L.; Li, X.; Li, C.; Fang, J. Ultraviolet irradiation of permanganate enhanced the oxidation of micropollutants by producing HO• and reactive manganese species. Environ. Sci. Technol. Lett. 2018, 5, 750–756. [Google Scholar] [CrossRef]
- Wei, W.; Guo, K.; Kang, X.; Zhang, J.; Li, C.; Fang, J. Complete removal of organoarsenic by the UV/Permanganate process via HO• oxidation and in situ-formed manganese dioxide adsorption. ACS EST Eng. 2021, 1, 794–803. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, Z.; Xue, X.; Yang, X.; Hua, L.; Fan, D. Killing effects of hydroxyl radical on algae and bacteria in ship’s ballast water and on their cell morphology. Plasma Chem. Plasma Process. 2010, 30, 831–840. [Google Scholar] [CrossRef]
- Chang, C.W.; Huo, X.; Lin, T.F. Exposure of Microcystis aeruginosa to hydrogen peroxide and titanium dioxide under visible light conditions: Modeling the impact of hydrogen peroxide and hydroxyl radical on cell rupture and microcystin degradation. Water Res. 2018, 141, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Petrusevski, V.; Van Breemen, A.; Alaerts, G. Effect of permanganate pre-treatment and coagulation with dual coagulants on algae removal in direct filtration. J. Water Supply Res. Technol. AQUA 1996, 45, 316–326. [Google Scholar]
- Fan, J.; Zeng, J.; Li, X.; Guo, K.; Liu, W.; Fang, J. Multiple roles of UV/KMnO4 in cyanobacteria containing water treatment: Cell inactivation & removal, and microcystin degradation. J. Hazard. Mater. 2023, 457, 131772. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.; Parsons, S.A.; Jefferson, B. The impact of algal properties and pre-oxidation on solid–liquid separation of algae. Water Res. 2008, 42, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, Q. Responses of bloom-forming Heterosigma akashiwo to allelochemical linoleic acid: Growth inhibition, oxidative stress and apoptosis. Front. Mar. Sci. 2022, 8, 793567. [Google Scholar] [CrossRef]
- Qu, F.; Du, X.; Liu, B.; He, J.; Ren, N.; Li, G.; Liang, H. Control of ultrafiltration membrane fouling caused by Microcystis cells with permanganate preoxidation: Significance of in situ formed manganese dioxide. Chem. Eng. J. 2015, 279, 56–65. [Google Scholar] [CrossRef]
- Strock, J.S.; Menden-Deuer, S. Temperature acclimation alters phytoplankton growth and production rates. Limnol. Oceanogr. 2021, 66, 740–752. [Google Scholar] [CrossRef]
- Markina, Z.V. The cell ultrastructure and autotrophic function of the raphidophyte alga Heterosigma akashiwo (Y. Hada) Y. Hada ex Y. Hara and M. Chihara, 1987 under Copper Exposure. Russ. J. Mar. Biol. 2021, 47, 204–209. [Google Scholar] [CrossRef]
- Okamoto, T.; Kim, D.; Oda, T.; Matsuoka, K.; Ishimatsu, A.; Muramatsu, T. Concanavalin a-Induced discharge of glycocalyx of raphidophycean flagellates, Chattonella marina and Heterosigma akashiwo. Biosci. Biotechnol. Biochem. 2000, 64, 1767–1770. [Google Scholar] [CrossRef]
- Jürgens, U.J.; Martin, C.; Weckesser, J. Cell wall constituents of Microcystis sp. PCC 7806. FEMS Microbiol. Lett. 1989, 65, 47–51. [Google Scholar] [CrossRef]
- Saber, H.; El-Sheekh, M.M.; Ibrahim, A.; Alwaleed, E.A. Effect of UV-B radiation on amino acids profile, antioxidant enzymes and lipid peroxidation of some cyanobacteria and green algae. Int. J. Radiat. Biol. 2020, 96, 1192–1206. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Dong, M.M.; Rosario-Ortiz, F.L. Using digital flow cytometry to assess the degradation of three cyanobacteria species after oxidation processes. Water Res. 2013, 47, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Rahn, R.O. Potassium iodide as a chemical actinometer for 254 nm radiation: Use of iodate as an electron scavenger. Photochem. Photobiol. 1997, 66, 885. [Google Scholar] [CrossRef]
- Fan, J.; Rao, L.; Chiu, Y.-T.; Lin, T.-F. Impact of chlorine on the cell integrity and toxin release and degradation of colonial Microcystis. Water Res. 2016, 102, 394–404. [Google Scholar] [CrossRef]
- Lin, S.; Yu, X.; Fang, J.; Fan, J. Influences of the micropollutant erythromycin on cyanobacteria treatment with potassium. Water Res. 2020, 177, 115786. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Ding, A.; Qu, F.; Shao, S.; Liu, B.; Wang, H.; Wu, D.; Li, G. Effects of pre-ozonation on the ultrafiltration of different natural organic matter (NOM) fractions: Membrane fouling mitigation, prediction and mechanism. J. Membr. Sci. 2016, 505, 15–25. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Plant Physiol. Biochem. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Strickland, J.; Parsons, T. A practical handbook of seawater analysis. Bull. Fish. Res. Board Can. 1968, 167, 185–194. [Google Scholar]
- Chen, B.; Zhao, L.; Yu, Q.J. Toxicological effects of hypoxanthine on Heterosigmaakashiwo: Mechanism of growth inhibition and change in hemolytic toxin content. Ecotox. Environ. Saf. 2021, 226, 112797. [Google Scholar] [CrossRef]
- Xu, H.; Pei, H.; Xiao, H.; Li, X.; Ma, C.; Hu, W. Inactivation of Microcystis aeruginosa by hydrogen-terminated porous Si wafer: Performance and mechanisms. J. Photochem. Photobiol. Biol. 2016, 158, 23–29. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Daly, R.; Hobson, P.; Ho, L.; Brookes, J. Impact of potassium permanganate on cyanobacterial cell integrity and toxin release and degradation. Chemosphere 2013, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Gao, N.; Deng, Y.; Qiao, J.; Wang, H. Immediate and long-term impacts of UV-C irradiation on photosynthetic capacity, survival and microcystin-LR release risk of Microcystis aeruginosa. Water Res. 2012, 46, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Gao, N.; Wei, C.; Deng, Y.; Qiao, J. Immediate and long-term impacts of potassium permanganate on photosynthetic activity, survival and microcystin-LR release risk of Microcystis aeruginosa. J. Hazard. Mater. 2012, 219–220, 267–275. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.; He, M.; Li, Y.; Ding, J.; Zhong, Y.; Zhang, H. Selective production of singlet oxygen for harmful cyanobacteria inactivation and cyanotoxins degradation: Efficiency and mechanisms. J. Hazard. Mater. 2023, 441, 129940. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Singh, P.R.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Mechanisms of Photoprotection in Cyanobacteria. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 145–171. [Google Scholar]
- Zhang, C.; Yi, Y.-L.; Hao, K.; Liu, G.-L.; Wang, G.-X. Algicidal activity of Salvia miltiorrhiza Bung on Microcystis aeruginosa—Towards identification of algicidal substance and determination of inhibition mechanism. Chemosphere 2013, 93, 997–1004. [Google Scholar] [CrossRef]
- Li, D.; Cong, W.; Cai, Z.; Shi, D.; Ouyang, F. Some physiological and biochemical changes in marine eukaryotic red tide alga Heterosigma akashiwo during the alleviation from iron limitation. Plant Physiol. Biochem. 2003, 41, 295–301. [Google Scholar] [CrossRef]
- Fan, G.; Bao, M.; Wang, B.; Wu, S.; Luo, L.; Li, B.; Lin, J. Inhibitory effects of Cu2O/SiO2 on the growth of Microcystis aeruginosa and Its mechanism. Nanomaterials 2019, 9, 1669. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Wang, W.; Liao, P.; Li, G.; Chen, H.; Cen, J.; Lu, S.; Wong, P.K.; An, T. Photocatalytic inactivation and destruction of harmful microalgae Karenia mikimotoi under visible-light irradiation: Insights into physiological response and toxicity assessment. Environ. Res. 2021, 198, 111295. [Google Scholar] [CrossRef]
- Li, L.; Shao, C.; Lin, T.-F.; Shen, J.; Yu, S.; Shang, R.; Yin, D.; Zhang, K.; Gao, N. Kinetics of cell Inactivation, toxin release, and degradation during permanganation of Microcystis aeruginosa. Environ. Sci. Technol. 2014, 48, 2885–2892. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, J.; Hu, Y.; Wang, L.; Zhang, L.; Zhou, Q.; Gao, N. Pre-oxidation with KMnO4 changes extra-cellular organic matter’s secretion characteristics to improve algal removal by coagulation with a low dosage of polyaluminium chloride. J. Environ. Sci. China 2013, 25, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, L.; Zhang, D.; Zhao, X.; Huang, L. Accelerating the decomposition of KMnO4 by photolysis and auto-catalysis: A green approach to synthesize a layered birnessite-type MnO2 assembled hierarchical nanostructure. RSC Adv. 2016, 6, 14192–14198. [Google Scholar] [CrossRef]
- Park, K.Y.; Choi, S.Y.; Ahn, S.K.; Kweon, J.H. Disinfection by-product formation potential of algogenic organic matter from Microcystis aeruginosa: Effects of growth phases and powdered activated carbon adsorption. J. Hazard. Mater. 2021, 408, 124864. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lan, H.; Liu, R.; Liu, H.; Qu, J. Fe(II)-regulated moderate pre-oxidation of Microcystis aeruginosa and formation of size-controlled algae flocs for efficient flotation of algae cell and organic matter. Water Res. 2018, 137, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rao, D.; Dong, H.; Sun, B.; Shao, B.; Cao, G.; Guan, X. The role of active manganese species and free radicals in permanganate/bisulfite process. J. Hazard. Mater. 2020, 388, 121735. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Crittenden, J. Linear free energy relationships between aqueous phase hydroxyl radical reaction rate constants and free energy of activation. Environ. Sci. Technol. 2011, 45, 3479–3486. [Google Scholar] [CrossRef]
- Ling, C.; Trick, C.G. Expression and standardized measurement of hemolytic activity in Heterosigma akashiwo. Harmful Algae 2010, 9, 522–529. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, B.; Zhao, L. Effect of algicidal compound Nomega-acetylhistamine on physiological response and algal toxins in Heterosigma akashiwo. Ecotoxicol. Environ. Saf. 2021, 208, 111423. [Google Scholar] [CrossRef]
- Enriquez, R.; Pichat, P. Interactions of humic acid, quinoline, and TiO2 in water in relation to quinoline photocatalytic removal. Langmuir 2001, 17, 6132–6137. [Google Scholar] [CrossRef]
- Westerhoff, P.; Aiken, G.; Amy, G.; Debroux, J. Relationships between the structure of natural organic matter and its reactivity towards molecular ozone and hydroxyl radicals. Water Res. 1999, 33, 2265–2276. [Google Scholar] [CrossRef]
- Chen, J.J.; Yeh, H.H. The mechanisms of potassium permanganate on algae removal. Water Res. 2005, 39, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W.; Ren, P.; Li, W.; Yang, X.; Zhou, J.; Li, J.; Li, Z.; Wang, D. Effective removal of diatoms (Synedra sp.) by pilot-scale UV/chlorine-flocculation process. Sep. Purif. Technol. 2022, 302, 122117. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Qiang, Z.; Qu, J.; Li, G.; Wang, D. Effects of calcium ions on surface characteristics and adsorptive properties of hydrous manganese dioxide. J. Colloid Interface Sci. 2009, 331, 275–280. [Google Scholar] [CrossRef]
- Pilson, M.E.Q. An Introduction to the Chemistry of the Sea, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Chen, X.; Jin, S.; Fan, J. Efficient Inactivation and Removal of a Harmful Marine Algae—Heterosigma akashiwo—By UV-Assisted Permanganate Oxidation. Water 2023, 15, 3633. https://doi.org/10.3390/w15203633
Zeng J, Chen X, Jin S, Fan J. Efficient Inactivation and Removal of a Harmful Marine Algae—Heterosigma akashiwo—By UV-Assisted Permanganate Oxidation. Water. 2023; 15(20):3633. https://doi.org/10.3390/w15203633
Chicago/Turabian StyleZeng, Jianwei, Xuegang Chen, Shidi Jin, and Jiajia Fan. 2023. "Efficient Inactivation and Removal of a Harmful Marine Algae—Heterosigma akashiwo—By UV-Assisted Permanganate Oxidation" Water 15, no. 20: 3633. https://doi.org/10.3390/w15203633
APA StyleZeng, J., Chen, X., Jin, S., & Fan, J. (2023). Efficient Inactivation and Removal of a Harmful Marine Algae—Heterosigma akashiwo—By UV-Assisted Permanganate Oxidation. Water, 15(20), 3633. https://doi.org/10.3390/w15203633