Solutions to Avoid False Positives for Rituximab in Pre-Transplant Crossmatches
Abstract
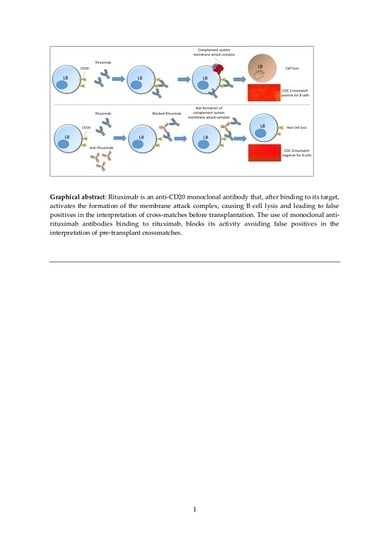
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HLA Typing and Anti-HLA Assays
2.3. Flow Cytometry Crossmatch
2.4. Complement-Dependent Cytotoxicity Crossmatch
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical approval
References
- Gürcan, H.M.; Keskin, D.B.; Stern, J.N.; Nitzberg, M.A.; Shekhani, H.; Ahmed, A.R. A review of the current use of rituximab in autoimmune diseases. Int. Immunopharmacol. 2009, 9, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Mehta, A.A. Rituximab in kidney disease and transplant. Anim. Models Exp. Med. 2019, 2, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Choi, J.; Vo, A. Kidney transplantation in highly sensitized patients. Br. Med. Bull. 2015, 114, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Book, B.K.; Pescovitz, M.D.; Guo, L.; Wiebke, E.A. Depletion of the chimeric drug Rituximab from biological samples. Hum. Immunol. 2017, 78, 500–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvezzi, P.; Senoussi, O.; Jouve, T.; de Palma, C.; Rostaing, L.; Bardy, B.; Masson, D. Rituximab interference in B-cell crossmatch: A problem solved. Am. J. Transpl. 2018, 18, 454. [Google Scholar]
- Sabatino, J.J., Jr.; Wilson, M.R.; Calabresi, P.A.; Hauser, S.L.; Schneck, J.P.; Zamvil, S.S. Anti-CD20 therapy depletes activated myelin-specific CD8(+) T cells in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 25800–25807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reindl-Schwaighofer, R.; Oberbauer, R. False-positive CDC x-match after Rituximab. Transpl. Int. 2014, 27, e124–e125. [Google Scholar] [CrossRef] [PubMed]
- Renaudineau, Y.; Devauchelle-Pensec, V.; Hanrotel, C.; Pers, J.; Saraux, A.; Youinou, P. Monoclonal anti-CD20 antibodies: Mechanisms of action and monitoring of biological effects. Jt. Bone Spine 2009, 76, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Alheim, M.; Wennberg, L.; Wikström, A. Pronase independent flow cytometry crossmatching of rituximab treated patients. Hum. Immunol. 2018, 79, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, S.J.; Schillinger, K.P.; Zachary, A.A.; Jackson, A.M. Impact of pronase on flow cytometric crossmatch outcome. Hum. Immunol. 2011, 72, 330–336. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex, Age (y/o) | Underlying Disease | Rituximab Dose | Anti-HLA II | SMCF B Cells | CDCXM B Cells Ω |
|---|---|---|---|---|---|---|
| 1 | F, 52 | RA | 500 mg/6 mo | No | a. 2806 (+) | a. 8 (+) |
| b. 178 (−) | b. 1 (−) | |||||
| 2 | M, 57 | RA | 500 mg/6 mo | No | a. 3250 (+) | a. 8 (+) |
| b. 230 (−) | b. 1 (−) | |||||
| 3 | F, 79 | RA, SSS | 500 mg/6 mo * | No | a. 2460 (+) | a. 8 (+) |
| b. 150 (−) | b. 1 (−) | |||||
| 4 | F, 68 | RA | 500 mg/6 mo ∫ | No | a. 2004 (+) | a. 8 (+) |
| b. 123 (−) | b. 1 (−) | |||||
| 5 | M, 50 | RA | 500 mg/6 mo | No | a. 2621 (+) | a. 8 (+) |
| b. 146 (−) | b. 1 (−) | |||||
| 6 | M, 30 | HT-1 | 660 mg | Yes | a. 7500 (+) | a. 8 (+) |
| b. 1430 (+) | b. 6 (+) | |||||
| 7 | F, 51 | IgAN | 500 mg | Yes | a. 8132 (+) | a. 8 (+) |
| b. 1492 (+) | b. 4–6 (+) | |||||
| 8 | F, 59 | NFG | 1000 mg ∫ | Yes | a. 6745 (+) | a. 8 (+) |
| b. 1115 (+) | b. 8 (+) | |||||
| 9 | M, 49 | PEGN ANCA | 750 mg | Yes | a. 7941 (+) | a. 8 (+) |
| b. 1522 (+) | b. 4–6 (+) | |||||
| 10 | M, 70 | PKD | 375 mg/m2 | Yes | a. 8982 (+) | a. 8 (+) |
| b. 1662 (+) | b. 6 (+) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmenero Velazquez, A.; Iturrieta-Zuazo, I.; Valdivieso Shephard, J.L.; Di Natale, M.; Rita, C.; Ballester González, R.; Castañer Alabau, J.L.; Nieto Gañán, I. Solutions to Avoid False Positives for Rituximab in Pre-Transplant Crossmatches. Antibodies 2020, 9, 41. https://doi.org/10.3390/antib9030041
Colmenero Velazquez A, Iturrieta-Zuazo I, Valdivieso Shephard JL, Di Natale M, Rita C, Ballester González R, Castañer Alabau JL, Nieto Gañán I. Solutions to Avoid False Positives for Rituximab in Pre-Transplant Crossmatches. Antibodies. 2020; 9(3):41. https://doi.org/10.3390/antib9030041
Chicago/Turabian StyleColmenero Velazquez, Argentina, Ignacio Iturrieta-Zuazo, Juan Luis Valdivieso Shephard, Marisa Di Natale, Claudia Rita, Rubén Ballester González, José Luis Castañer Alabau, and Israel Nieto Gañán. 2020. "Solutions to Avoid False Positives for Rituximab in Pre-Transplant Crossmatches" Antibodies 9, no. 3: 41. https://doi.org/10.3390/antib9030041
APA StyleColmenero Velazquez, A., Iturrieta-Zuazo, I., Valdivieso Shephard, J. L., Di Natale, M., Rita, C., Ballester González, R., Castañer Alabau, J. L., & Nieto Gañán, I. (2020). Solutions to Avoid False Positives for Rituximab in Pre-Transplant Crossmatches. Antibodies, 9(3), 41. https://doi.org/10.3390/antib9030041