Monoclonal Antibodies Generated against Glycoconjugates Recognize Chemical Linkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Parasite Extracts
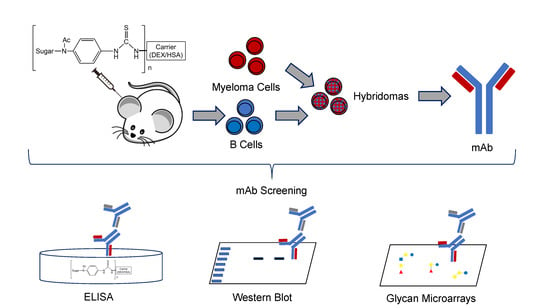
2.2. Mouse Immunizations
2.3. Cell Fusion, Hybridoma Selection, & Screening
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. mAb Purification
2.6. RAW 264.7 Cell Culture
2.7. Characterization of Monoclonal Antibody
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, C.; Wei, M.; McKitrick, T.R.; McQuillan, A.M.; Heimburg-Molinaro, J.; Cummings, R.D. Glycan microarrays as chemical tools for identifying glycan recognition by immune proteins. Front. Chem. 2019, 7, 833. [Google Scholar] [CrossRef] [Green Version]
- Varki, A. Biological roles of glycans. Glycobiology 2016, 27, 3–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copoiu, L.; Malhotra, S. The current structural glycome landscape and emerging technologies. Curr. Opin. Struct. Biol. 2020, 62, 132–139. [Google Scholar] [CrossRef]
- Haab, B.B.; Klamer, Z. Advances in tools to determine the glycan-binding specificities of lectins and antibodies. Mol. Cell. Proteom. 2020, 19, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura-Tsuruta, S.; Kominami, J.; Kuno, A.; Hirabayashi, J. Evidence that Agaricus bisporus agglutinin (ABA) has dual sugar-binding specificity. Biochem. Biophys. Res. Commun. 2006, 347, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Higashida, K.; Hata, Y.; Kominami, J.; Nakamura-Tsuruta, S.; Hirabayashi, J. Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Anal. Biochem. 2009, 386, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lei, Z.; Liu, D.; Wang, Z. Development of a sandwiched microarray platform for studying the interactions of antibiotics with Staphylococcus aureus. Anal. Chim. Acta 2016, 917, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-L.; Pilobello, K.T.; Mahal, L.K. Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nat. Chem. Biol. 2006, 2, 153–157. [Google Scholar] [CrossRef]
- Kilcoyne, M.; Twomey, M.E.; Gerlach, J.Q.; Kane, M.; Moran, A.P.; Joshi, L. Campylobacter jejuni strain discrimination and temperature-dependent glycome expression profiling by lectin microarray. Carbohydr. Res. 2014, 389, 123–133. [Google Scholar] [CrossRef]
- Yasuda, E.; Tateno, H.; Hirabarashi, J.; Iino, T.; Sako, T. Lectin microarray reveals binding profiles of lactobacillus casei strains in a comprehensive analysis of bacterial cell wall polysaccharides. Appl. Environ. Microbiol. 2011, 77, 4539–4546. [Google Scholar] [CrossRef] [Green Version]
- Dang, K.; Zhang, W.; Jiang, S.; Lin, X.; Qian, A. Application of lectin microarrays for biomarker discovery. ChemistryOpen 2020, 9, 285–300. [Google Scholar] [CrossRef]
- Cummings, R.D.; Darvill, A.G.; Etzler, M.E.; Hahn, M.G. Glycan-recognizing probes as tools. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 611–625. [Google Scholar] [CrossRef]
- Mandalasi, M.; Dorabawila, N.; Smith, D.F.; Heimburg-Molinaro, J.; Cummings, R.D.; Nyame, A.K. Development and characterization of a specific IgG monoclonal antibody toward the Lewis x antigen using splenocytes of Schistosoma mansoni-infected mice. Glycobiology 2013, 23, 877–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harn, D.A.; Mitsuyama, M.; David, J.R. Schistosoma mansoni. Anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J. Exp. Med. 1984, 159, 1371–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, A.I.; Harn, D.A. Characterization of protective and non-protective surface membrane carbohydrate epitopes of Schistosoma mansoni. Mem. Inst. Oswaldo Cruz 1987, 82 (Suppl. 4), 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harn, D.A.; Quinn, J.J.; Cianci, C.M.; Ko, A.I. Evidence that a protective membrane epitope is involved in early but not late phase immunity in Schistosoma mansoni. J. Immunol. 1987, 138, 1571–1580. [Google Scholar]
- Ko, A.I.; Drager, U.C.; Harn, D.A. A Schistosoma mansoni epitope recognized by a protective monoclonal antibody is identical to the stage-specific embryonic antigen 1. Proc. Natl. Acad. Sci. USA 1990, 87, 4159–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimburg-Molinaro, J.; Rittenhouse-Olson, K. Development and characterization of antibodies to carbohydrate antigens. In Glycomics: Methods and Protocols; Packer, N.H., Karlsson, N.G., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 341–357. [Google Scholar] [CrossRef]
- Solter, D.; Knowles, B.B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569. [Google Scholar] [CrossRef] [Green Version]
- Hokke, C.H.; Deelder, A.M. Schistosome glycoconjugates in host-parasite interplay. Glycoconj. J. 2001, 18, 573–587. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Palanivel, V.; Posey, C.; Horauf, A.M.; Solbach, W.; Piessens, W.F.; Harn, D.A. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp. Parasitol. 1996, 84, 168–177. [Google Scholar] [CrossRef]
- Atochina, O.; Daly-Engel, T.; Piskorska, D.; McGuire, E.; Harn, D.A. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naive CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J. Immunol. 2001, 167, 4293–4302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.G.; Carter, M.R.; Atochina, O.; Da’Dara, A.A.; Piskorska, D.; McGuire, E.; Harn, D.A. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 2003, 171, 5837–5841. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.G.; Carter, M.R.; Da’dara, A.A.; DeSimone, T.M.; Harn, D.A. A helminth glycan induces APC maturation via alternative NF-kappa B activation independent of I kappa B alpha degradation. J. Immunol. 2005, 175, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Li, C.; Stanya, K.J.; Jacobi, D.; Dai, L.; Liu, S.; Gangl, M.R.; Harn, D.A.; Lee, C.H. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat. Med. 2012, 18, 1665–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tivadar, S.T.; McIntosh, R.S.; Chua, J.X.; Moss, R.; Parsons, T.; Zaitoun, A.M.; Madhusudan, S.; Durrant, L.G.; Vankemmelbeke, M. Monoclonal antibody targeting sialyl-di-Lewisa–containing internalizing and noninternalizing glycoproteins with cancer immunotherapy development potential. Mol. Cancer Ther. 2020, 19, 790–801. [Google Scholar] [CrossRef] [Green Version]
- De Rougemont, A.; Ruvoen-Clouet, N.; Simon, B.; Estienney, M.; Elie-Caille, C.; Aho, S.; Pothier, P.; Le Pendu, J.; Boireau, W.; Belliot, G. Qualitative and quantitative analysis of the binding of GII.4 norovirus variants onto human blood group antigens. J. Virol. 2011, 85, 4057–4070. [Google Scholar] [CrossRef] [Green Version]
- Grenfell, R.F.; Shollenberger, L.M.; Samli, E.F.; Harn, D.A. Vaccine self-assembling immune matrix is a new delivery platform that enhances immune responses to recombinant HBsAg in mice. Clin. Vaccine Immunol. 2015, 22, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Fishman, J.B.; Berg, E.A. Ammonium sulfate fractionation of antibodies. Cold Spring Harb. Protoc. 2018, 2018. [Google Scholar] [CrossRef]
- Yu, Y.; Mishra, S.; Song, X.; Lasanajak, Y.; Bradley, K.C.; Tappert, M.M.; Air, G.M.; Steinhauer, D.A.; Halder, S.; Cotmore, S.; et al. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J. Biol. Chem. 2012, 287, 44784–44799. [Google Scholar] [CrossRef] [Green Version]
- Velupillai, P.; Harn, D.A. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: A mechanism for regulation of CD4+ T-cell subsets. Proc. Natl. Acad. Sci. USA 1994, 91, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atochina, O.; Harn, D. LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin. Diagn. Lab. Immunol. 2005, 12, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atochina, O.; Da’dara, A.A.; Walker, M.; Harn, D.A. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology 2008, 125, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, L.; Tundup, S.; Choi, B.S.; Norberg, T.; Harn, D. Immunomodulatory glycan lacto-N-fucopentaose III requires clathrin-mediated endocytosis to induce alternative activation of antigen-presenting cells. Infect. Immun. 2014, 82, 1891–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundup, S.; Srivastava, L.; Nagy, T.; Harn, D. CD14 influences host immune responses and alternative activation of macrophages during Schistosoma mansoni infection. Infect. Immun. 2014, 82, 3240–3251. [Google Scholar] [CrossRef] [Green Version]
- Atochina, O.; Harn, D. Prevention of psoriasis-like lesions development in fsn/fsn mice by helminth glycans. Exp. Dermatol. 2006, 15, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Trikudanathan, S.; Zozulya, A.L.; Sandoval-Garcia, C.; Kennedy, J.K.; Atochina, O.; Norberg, T.; Castagner, B.; Seeberger, P.; Fabry, Z.; et al. Immune modulation by Lacto-N-fucopentaose III in experimental autoimmune encephalomyelitis. Clin. Immunol. 2012, 142, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.; Hullett, D.A.; Roenneburg, D.A.; Torrealba, J.R.; Sollinger, H.W.; Harn, D.A.; Burlingham, W.J. Lacto-N-fucopentaose III, a pentasaccharide, prolongs heart transplant survival. Transplantation 2010, 90, 1071–1078. [Google Scholar] [CrossRef]








| Purification Step | Protein [mg/mL] | 260/280 Ratio |
|---|---|---|
| Pre-Ammonium Sulfate | 4.93 | 0.83 |
| Post-25% Ammonium Sulfate | 3.76 | 0.83 |
| Post-50% Ammonium Sulfate | 3.19 | 0.96 |
| Post-Dialysis | 2.64 | 0.85 |
| Post-G Column Purification | 1.79 | 0.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadhin, J.; Silva-Moraes, V.; Norberg, T.; Harn, D. Monoclonal Antibodies Generated against Glycoconjugates Recognize Chemical Linkers. Antibodies 2020, 9, 48. https://doi.org/10.3390/antib9030048
Ramadhin J, Silva-Moraes V, Norberg T, Harn D. Monoclonal Antibodies Generated against Glycoconjugates Recognize Chemical Linkers. Antibodies. 2020; 9(3):48. https://doi.org/10.3390/antib9030048
Chicago/Turabian StyleRamadhin, Jessica, Vanessa Silva-Moraes, Thomas Norberg, and Donald Harn. 2020. "Monoclonal Antibodies Generated against Glycoconjugates Recognize Chemical Linkers" Antibodies 9, no. 3: 48. https://doi.org/10.3390/antib9030048
APA StyleRamadhin, J., Silva-Moraes, V., Norberg, T., & Harn, D. (2020). Monoclonal Antibodies Generated against Glycoconjugates Recognize Chemical Linkers. Antibodies, 9(3), 48. https://doi.org/10.3390/antib9030048