Synthesis and Reactivities of Triphenyl Acetamide Analogs for Potential Nonlinear Optical Material Uses
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Computational Studies
2.2.1. Geometry Optimization
2.2.2. Frontier Molecular Orbital (FMO) Analysis
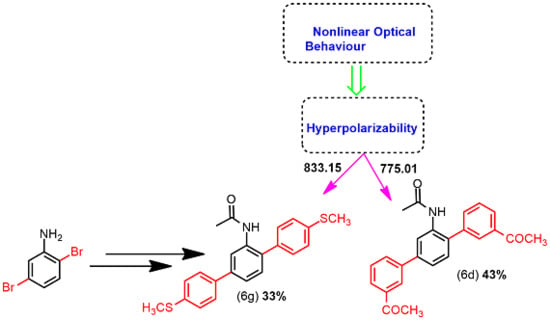
2.2.3. Nonlinear Optical Properties (NLO)
2.2.4. Conceptual Reactivity Descriptors
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of N-(2,5-Dibromophenyl)acetamide (2)
3.3. General Procedure for the Synthesis of Compound (3a–h)
3.4. Characterization Data
3.5. Computational Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.J.; Wang, H.; Tino, J.A.; Robl, J.A.; Herpin, T.F.; Lawrence, R.M.; Biller, S.; Jamil, H.; Ponticiello, R.; Chen, L.; et al. 2-Hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorgan. Med. Chem. Lett. 2007, 17, 3208–3211. [Google Scholar] [CrossRef]
- Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti, O.; Rieger, B. New nickel (II) diimine complexes and the control of polyethylene microstructure by catalyst design. J. Am. Chem. Soc. 2007, 129, 9182–9191. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H.; Ismael, R.; Yürük, O. Persistent fluorescence of perylene dyes by steric inhibition of aggregation. Tetrahedron 2000, 56, 5435–5441. [Google Scholar] [CrossRef]
- Zakai, U.I.; Błoch-Mechkour, A.; Jacobsen, N.E.; Abrell, L.; Lin, G.; Nichol, G.S.; Bally, T.; Glass, R.S. Synthesis and structure of m-terphenyl thio-, seleno-, and telluroethers. J. Organ. Chem. 2010, 75, 8363–8371. [Google Scholar] [CrossRef] [PubMed]
- Zuideveld, M.A.; Wehrmann, P.; Röhr, C.; Mecking, S. Remote substituents controlling catalytic polymerization by very active and robust neutral nickel (II) complexes. Angew. Chem. 2004, 116, 887–891. [Google Scholar] [CrossRef]
- Alexander, S.G.; Cole, M.L.; Morris, J.C. Preparation of a super bulky silver N-heterocyclic carbene complex. New J. Chem. 2009, 33, 720–724. [Google Scholar] [CrossRef]
- Eberhardt, R.; Allmendinger, M.; Luinstra, G.A.; Rieger, B. The ethylsulfinate ligand: A highly efficient initiating group for the zinc β-diiminate catalyzed copolymerization reaction of CO2 and epoxides. Organometallics 2003, 22, 211–214. [Google Scholar] [CrossRef]
- Bai, G.; Singh, S.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G. Mononuclear aluminum hydroxide for the design of well-defined homogeneous catalysts. J. Am. Chem. Soc. 2005, 127, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Jin, M.-J. An extremely active and general catalyst for Suzuki coupling reaction of unreactive aryl chlorides. Organ. Lett. 2010, 13, 252–255. [Google Scholar] [CrossRef]
- Iqbal, J.; Tirmizi, S.A.; Wattoo, F.H.; Imran, M.; Wattoo, M.H.S.; Sharfuddin, S.; Latif, S. Biological properties of chloro-salicylidene aniline and its complexes with Co (II) and Cu (II). Turk. J. Biol. 2006, 30, 1–4. [Google Scholar]
- Gizdavic-Nikolaidis, M.R.; Bennett, J.R.; Swift, S.; Easteal, A.J.; Ambrose, M. Broad spectrum antimicrobial activity of functionalized polyanilines. Acta Biomater. 2011, 7, 4204–4209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-K.; Guan, J.; Tachibana, Y.; Bastow, K.F.; Cho, S.J.; Cheng, H.-H.; Cheng, Y.-C.; Gurwith, M.; Lee, K.-H. Antitumor Agents. 194. Synthesis and biological evaluations of 4-β-mono-,-di-, and-trisubstituted aniline-4′-O-demethyl-podophyllotoxin and related compounds with improved pharmacological profiles. J. Med. Chem. 1999, 42, 2441–2446. [Google Scholar] [CrossRef]
- Jangid, N.K.; Chauhan, N.P.S.; Meghwal, K.; Ameta, R.; Punjabi, P.B. Synthesis of dye-substituted polyanilines and study of their conducting and antimicrobial behavior. Cogent Chem. 2015, 1, 1084666. [Google Scholar] [CrossRef]
- Yılmaz, K.; Akgoz, A.; Cabuk, M.; Karaagac, H.; Karabulut, O.; Yavuz, M. Electrical transport, optical and thermal properties of polyaniline–pumice composites. Mater. Chem. Phys. 2011, 130, 956–961. [Google Scholar] [CrossRef]
- Child, R.; Wilkinson, R.; Tomcu-Fucik, A. Effect of Substrate orientation of the adhesion of polymer joints. Chem. Abstr. 1977, 1977, 6031. [Google Scholar]
- Garg, H.; Prakash, C. Potential antidiabetics. 11. Preparation of 4-arylazo-3, 5-disubstituted-(2H)-1, 2, 6-thiadiazine 1, 1-dioxides. J. Med. Chem. 1972, 15, 435–436. [Google Scholar] [CrossRef]
- Rathod, K.; Thakre, N. Synthesis and antimicrobial activity of azo compounds containing m-cresol moiety. Chem. Sci. Trans. 2013, 2, 25–28. [Google Scholar] [CrossRef]
- Park, C.; Lim, J.-S.; Lee, Y.; Lee, B.; Kim, S.-W.; Lee, J.; Kim, S. Optimization and morphology for decolorization of reactive black 5 by Funalia trogii. Enzyme Microb. Technol. 2007, 40, 1758–1764. [Google Scholar] [CrossRef]
- Swati, G.; Romila, K.; Sharma, I.; Verma, P. Synthesis, characterization and antimicrobial screening of some azo compounds. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 332–338. [Google Scholar]
- Martin, A.R.; Yang, Y. Palladium-catalyzed cross-coupling reactions of organoboronic acids with organic electrophiles. Acta Chem. Scand. 1993, 47, 221–230. [Google Scholar] [CrossRef]
- Suzuki, A. New synthetic transformations via organoboron compounds. Pure Appl. Chem. 1994, 66, 213–222. [Google Scholar] [CrossRef]
- Stanforth, S.P. Catalytic cross-coupling reactions in biaryl synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Shi, J.-M.; Xu, W.; Liu, Q.-Y.; Liu, F.-L.; Huang, Z.-L.; Lei, H.; Yu, W.-T.; Fang, Q. Polynitrile-bridged two-dimensional crystal: Eu (III) complex with strong fluorescence emission and NLO property. Chem. Commun. 2002, 123, 756–757. [Google Scholar] [CrossRef]
- Chang, H.-L.; Lin, H.-L.; Wang, Y.-C.; Dai, S.A.; Su, W.-C.; Jeng, R.-J. Thermally stable NLO poly (amide–imide) s via sequential self-repetitive reaction. Polymer 2007, 48, 2046–2055. [Google Scholar] [CrossRef]
- Kolev, T.; Koleva, B.B.; Spassov, T.; Cherneva, E.; Spiteller, M.; Mayer-Figge, H.; Sheldrick, W.S. Synthesis, spectroscopic, thermal and structural elucidation of 5-amino-2-methoxypyridine ester amide of squaric acid ethyl ester: A new material with an infinite pseudo-layered structure and manifested NLO application. J. Mol. Struct. 2008, 875, 372–381. [Google Scholar] [CrossRef]
- Gull, Y.; Rasool, N.; Noreen, M.; Altaf, A.A.; Musharraf, S.G.; Zubair, M.; Nasim, F.-U.-H.; Yaqoob, A.; DeFeo, V.; Zia-Ul-Haq, M. Synthesis of N-(6-arylbenzo [d] thiazole-2-acetamide derivatives and their biological activities: An experimental and computational approach. Molecules 2016, 21, 266. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Ali, E.A.; Mokhtar, S.M.; Eweis, M. Synthesis, characterization polymerization and antibacterial properties of novel thiophene substituted acrylamide. React. Funct. Polym. 2011, 71, 1187–1194. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Lein, M. Carbon nano-onions as photosensitizers: Stacking-induced red-shift. J. Phys. Chem. C 2018, 122, 2422–2431. [Google Scholar] [CrossRef]
- Arshad, M.N.; Bibi, A.; Mahmood, T.; Asiri, A.M.; Ayub, K. Synthesis, crystal structures and spectroscopic properties of triazine-based hydrazone derivatives; A comparative experimental-theoretical study. Molecules 2015, 20, 5851–5874. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R. Organic nonlinear optical materials: Where we have been and where we are going. Chem. Commun. 2006, 37, 131–134. [Google Scholar] [CrossRef]
- Champagne, B.; Plaquet, A.; Pozzo, J.-L.; Rodriguez, V.; Castet, F. Nonlinear optical molecular switches as selective cation sensors. J. Am. Chem. Soc. 2012, 134, 8101–8103. [Google Scholar] [CrossRef]
- Burland, D. Optical nonlinearities in chemistry: Introduction. Chem. Rev. 1994, 94, 1–2. [Google Scholar] [CrossRef]
- Nayak, P.K.; Periasamy, N. Calculation of electron affinity, ionization potential, transport gap, optical band gap and exciton binding energy of organic solids using ‘solvation’ model and DFT. Org. Electron. 2009, 10, 1396–1400. [Google Scholar] [CrossRef]
- Zhan, C.-G.; Nichols, J.A.; Dixon, D.A. Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: Molecular properties from density functional theory orbital energies. J. Phys. Chem. A 2003, 107, 4184–4195. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Cammi, R.; Mennucci, B.; Tomasi, J. Fast evaluation of geometries and properties of excited molecules in solution: A Tamm-Dancoff Model with application to 4-dimethylaminobenzonitrile. J. Phys. Chem. A 2000, 104, 5631–5637. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Solvent effect on vertical electronic transitions by the polarizable continuum model. J. Chem. Phys. 2000, 112, 2427–2435. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Time-dependent density functional theory for molecules in liquid solutions. J. Chem. Phys. 2001, 115, 4708–4717. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Polarizable dielectric model of solvation with inclusion of charge penetration effects. J. Chem. Phys. 2001, 114, 5691–5701. [Google Scholar] [CrossRef]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J. Chem. Phys. 2002, 117, 43–54. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- CYLview, B.; Legault, C.Y. Université de Sherbrooke. 2009. Available online: http://www.cylview.org (accessed on 12 January 2019).




| Compound | EHOMO (eV) | ELUMO (eV) | H-LEg (eV) | βo (au) |
|---|---|---|---|---|
| 3a | −6.17 | −1.15 | 5.02 | 236.99 |
| 3b | −5.84 | −1.01 | 4.84 | 465.20 |
| 3c | −6.31 | −1.51 | 4.80 | 326.75 |
| 3d | −6.54 | −1.80 | 4.75 | 775.01 |
| 3e | −6.39 | −1.49 | 4.90 | 252.00 |
| 3f | −6.64 | −1.67 | 4.96 | 311.90 |
| 3g | −5.74 | −1.23 | 4.51 | 833.15 |
| 3h | −6.68 | −1.36 | 5.32 | 245.70 |
| Compound | Ionization Potential, I (eV) | Electron Affinity, (EA) (eV) | Chemical Hardness, ƞ (eV) | Electronic Chemical Potential, μ (eV) | Electrophilicity Index, ω (eV) |
|---|---|---|---|---|---|
| 3a | 6.17 | 1.15 | 2.51 | −3.66 | 2.67 |
| 3b | 5.84 | 1.01 | 2.42 | −3.42 | 2.42 |
| 3c | 6.31 | 1.51 | 2.40 | −3.91 | 3.18 |
| 3d | 6.54 | 1.80 | 2.37 | −4.17 | 3.67 |
| 3e | 6.39 | 1.49 | 2.45 | −3.94 | 3.17 |
| 3f | 6.64 | 1.67 | 2.48 | −4.16 | 3.48 |
| 3g | 5.74 | 1.23 | 2.26 | −3.48 | 2.69 |
| 3h | 6.68 | 1.36 | 2.66 | −4.02 | 3.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Israr, H.; Rasool, N.; Rizwan, K.; Hashmi, M.A.; Mahmood, T.; Rashid, U.; Hussein, M.Z.; Akhtar, M.N. Synthesis and Reactivities of Triphenyl Acetamide Analogs for Potential Nonlinear Optical Material Uses. Symmetry 2019, 11, 622. https://doi.org/10.3390/sym11050622
Israr H, Rasool N, Rizwan K, Hashmi MA, Mahmood T, Rashid U, Hussein MZ, Akhtar MN. Synthesis and Reactivities of Triphenyl Acetamide Analogs for Potential Nonlinear Optical Material Uses. Symmetry. 2019; 11(5):622. https://doi.org/10.3390/sym11050622
Chicago/Turabian StyleIsrar, Hira, Nasir Rasool, Komal Rizwan, Muhammad Ali Hashmi, Tariq Mahmood, Umer Rashid, Mohd Zobir Hussein, and Muhammad Nadeem Akhtar. 2019. "Synthesis and Reactivities of Triphenyl Acetamide Analogs for Potential Nonlinear Optical Material Uses" Symmetry 11, no. 5: 622. https://doi.org/10.3390/sym11050622
APA StyleIsrar, H., Rasool, N., Rizwan, K., Hashmi, M. A., Mahmood, T., Rashid, U., Hussein, M. Z., & Akhtar, M. N. (2019). Synthesis and Reactivities of Triphenyl Acetamide Analogs for Potential Nonlinear Optical Material Uses. Symmetry, 11(5), 622. https://doi.org/10.3390/sym11050622