1. Introduction
The fabrication of carbonaceous nanostructured materials is a challenging area of research. Carbon nanomaterials (CNMs), including carbon nanotubes (CNTs), carbon nanofibers (CNFs), and other CNM forms are still attracting great interest in the scientific and industrials communities for their superior surface characteristics and the potential for application in cutting edge technologies to solve environmental and technical challenges [
1,
2,
3,
4]. Although there is considerable development required for the fabrication and growth of CNTs, the large-scale synthesis of CNTs is still difficult and constrained by several factors.
Super-hydrophobic carbon nanomaterials (CNMs), which have contact angles (
CA) > 150°, play a significant role in tackling many technical problems related to the chemical and physical nature of surfaces and contacts. Therefore, super-hydrophobic CNMs have been involved in various applications, including drug delivery materials [
5], adsorbents [
6,
7], antifouling distillation membrane [
8], self-healing membranes [
9], and others [
10,
11]. The fabrication of nanomembrane containing CNMs for membrane distillation (MD) used for desalination depends on the vapor pressure gradients through the super-hydrophobic, carbonaceous, microporous membrane to ensure higher water flux with greater salt rejection rate [
8]. The application of CNMs can overall reduce energy consumption, fouling tendencies, and mitigate the frequent replacement of membrane up to a significant level. The physio-chemical properties, including roughness of the surface and surface functional groups, can significantly alter the hydrophobicity of the synthesized nanomaterials [
11]. Growing CNMs on other substrates can change the surface chemistry to a greater extent. It can even considerably alter the surface roughness of the synthesized nanoparticles [
10]. Therefore, to ensure appropriate surface roughness, as well as the necessary functional groups, over the CNM to retain its super hydrophobicity, an improved fabrication technique is required.
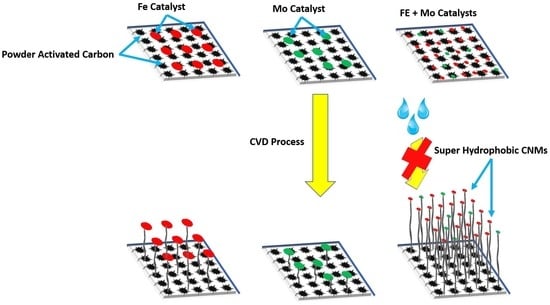
Until recently, CNMs have been synthesized using several methods, including template synthesis, chemical vapor deposition (CVD), crystallization control, electrochemical deposition, etc. [
12]. The CVD method is appropriate for the large-scale production of carbon nanomaterials using different carbonaceous sources and can be deposited using various types of transition metal (Fe, Co, and Ni) catalysts at higher temperatures (500–1200 °C). Among all the methods, CVD is considered as the most efficient one owing to its widespread accessibility, and simple operating procedure with higher product yield [
13,
14]. Silicon oxide and alumina and other oxides are the main conventional substrates utilized for the CVD process. However, to obtain highly pure carbon samples, further chemical purification processes are needed. Therefore, they are not suitable for certain environmental applications. Therefore, powder activated carbon (PAC) can be considered as a promising alternative precursor due to its lower cost, easy availability, and better possibility for surface modification by chemical treatments [
12,
15,
16]. Due to the variation in the types of PAC extracted from different sources as well as the variables for CVD process and catalyst types, the physiochemical properties of the synthesized nanocarbon samples can be unique and vary greatly across different samples. PAC was used earlier to obtain nano-fibrous carbon (CNFs) where iron (Fe) was used as catalyst in presence of ethylene (C
2H
4) gas flow during the CVD process [
12,
17,
18,
19,
20]. On the other hand, modification of CNM structure has been done by other researchers to achieve artificial super-hydrophobic surfaces. Optimization of the synthesis of super-hydrophobic CNMs could be hampered due to the aggregation property of CNMs and impurities [
21]. The hybrid PAC–CNMs maintain the chemical compatibility between these two materials and combine the characteristics of PAC and CNMs.
The quality and yield of CNMs produced by CVD can be enhanced by using an appropriate catalyst as it plays a substantial part in the synthesis process. The catalyst can initiate the decomposition of hydrocarbons at a lower temperature to accelerate new nucleation to form CNMs compared to the un-catalyzed hydrocarbon decomposition process using heat only. It was reported earlier that some metals such as Ni, Co, or Fe, which have few d-vacancies, are suitable catalyst for nucleation and growth of CNTs [
21,
22]. Application of appropriate chemical agent or catalyst having optimum ratio can produce CNTs with unique mechanical, physical, chemical, electrical, and thermal properties which are essential for efficient membrane separation [
21,
22].
Even Some other transition metals have a strong adhesion to grow CNMs as compared to others such as Fe, Ni, and Co, therefore they have been used efficiently to form high-curvature (low-diameter) CNMs [
23]. Other transition metals like Mo, Cu, Ag, Cr, Mn, and Pd are also used as catalysts for synthesis of CNMs [
24,
25]. It was found that not only growth rate and diameter are highly influenced by the catalyst type and composition, but also the CNM’s microstructure and morphology [
26]. Moreover, synthesis of CNMs from hydrocarbons has a synergistic catalytic effect with bimetallic catalysts [
27,
28,
29]. For example, the growth of CNMs was improved by using bimetallic catalysts such as Fe–Co, Co–Mo, and Fe–Ni [
30,
31,
32,
33,
34]. All of the above-mentioned catalysts (single or bimetallic catalysts) were successfully used with substrates other than PAC in most cases, due to the difficulties in formation of metallic nanocluster on the surface of PAC with conventional methods.
The conventional method for any chemical synthesis process by changing one variable at a time and keeping other variables at a fixed level cannot demonstrate the combined effect of all the variables on the desired output. Thus, multivariable processes needs a large number of experiments to be carried out, which is time consuming and not efficient enough to illustrate the interaction effect [
35]. In this context, the application of response surface methodology (RSM) using Box Behnken Design (BBD) is considered as an appropriate method which can systematically describe the linear, nonlinear, and interaction effect between the factors [
36]. In this research, the CVD process was optimized using BBD design. The combination of more than one catalyst at a time affects the characteristics of the grown CNMs due to the interaction between the substrates’ physiochemical nature and different types of metals’ behavior. To the best of our knowledge, until now, no research has been conducted to observe the effect of binary metal catalysts on the growth of nanostructured carbonaceous materials using powdered activated carbon (PAC) as a starting precursor. In this research work, the combination of a binary metal catalyst (Fe/Mo) is used and PAC is used as a starting precursor to produce super-hydrophobic CNMs which have unique features suitable for membrane fabrication and separation processes. The thermal CVD method was used to decompose acetylene as a carbon source at 650 °C. The Optimum Yield% of carbon and contact angle (
CA) were chosen as the output responses using the RSM approach. The goal of this study is to produce super-hydrophobic CNMs which can be used for several applications, such as sorption [
6,
37], membrane distillation [
38], separation of organic components in mixture, purification of water, and catalysis [
39].
2. Materials and Methods
2.1. Materials and Reagents
Iron nitrate Fe (NO3)3·9H2O, ammonium molybdate tetrahydrate (NH4)6Mo7O4·4H2O, PAC, and acetone were purchased from Sigma Aldrich, Malaysia. Acetylene gas (C2H2), hydrogen gas (H2), and nitrogen gas (N2) were purchased from GasLink Industrial Gases SDN BHD, Malaysia. Analytical grade reagents and chemicals were used in this research. Thus, no additional purification steps were necessary for conducting the experiments.
2.2. Synthesis of Binary Metal Catalyst
The bimetallic catalysts (iron and molybdenum) were first dissolved in acetone using their salts. Then incipient wetness method was used to deposit the catalyst over the surface of the PAC. Different weight percent (
w/
w%) samples of the catalysts (as shown in
Table 1) were prepared. The ratios between PAC and catalysts were calculated based on the design matrix provided by BBD design. According to
Table 1, the catalysts solution was prepared by dissolving the desired amount of the catalysts in 5 mL of acetone solution. The mixture was kept inside the universal glass bottle and stirred until all the catalysts salts were completely dissolved.
2.3. Impregnation Powdered Activated Carbon (PAC)
The metallic catalysts (iron and molybdenum) were impregnated on powdered activated carbon (PAC). After the catalysts were dissolved in acetone, PAC (2 g) was added into the same solvent in a glass bottle and placed in a sonication bath at 40 KHz and 60 °C for 6 h until all the solvent evaporated, then moved to a conventional oven at 100 °C overnight to ensure complete moisture evaporation. The dried sample thus obtained was finely grounded. The powdered mixture obtained was stored using a desiccator to prevent moisture adsorption and sent for further application.
2.4. Synthesis of Nanostructured Carbon (CNMs)
The desired ratio of catalysts and PAC was mixed and transferred to the ceramic boat (50 mm OD, 40 mm ID, 1500 mm L). This boat was then inserted inside the CVD reaction tube. Under inert atmosphere (N2, 200 mL/min), the sample was first calcinated at 350 °C for 2 h. An in situ reduction process of the calcined sample was carried out by passing hydrogen gas (200 mL/min) at 450 °C for another 2 h. The final product thus obtained was the reduced sample which was then cooled down to room temperature. At this stage, the amount of moisture present in the sample was determined by weighing the sample (WC).
The reduced catalyst mixture was placed inside the tubular ceramic reactor at atmospheric pressure where a mixture of acetylene (50 mL/min) and hydrogen (200 mL/min) gas was flown. For growth of CNMs, the acetylene to hydrogen gas ratio was kept at 1:4 and the reaction was carried out at 650 °C for different reaction time as provided by the design matrix. After the reaction was completed, the reactor was cooled down using N
2 gas flow (200 mL/min). As-synthesized CNMs were then collected from the ceramic boat and weighed (
WP). The carbon Yield% was calculated using Equation (1).
where
WP and
WC are the weight of sample after and before of the reaction, respectively.
2.5. Equipment and Measurements
All the weighing measurements were recorded using four digits weighing balance (HR-202i, Japan) in a range between 0.001 and 220 g. A predetermined weight of powdered activated carbon (PAC) was physically mixed with the catalyst solution and the resultant mixture was placed inside the ultrasonic bath model (JAC 2010 P, South Korea) to ensure proper impregnation of metal catalysts onto PAC substrate. It is equipped with three levels of sonication, a timer up to 99 min, and heater up to 90 °C. The drying of PAC and CNMs samples was carried out in a drying oven (Model 600-Memmert, Germany), where the maximum temperature can be set up to 220 °C. The CVD process was carried out using an OTF-1200-80 mm dual-zone tube furnace for growth of CNMs. The tube furnace contains a tubular reactor which is equipped with a fused quartz tube with a diameter of 50 mm (OD: 80 mm; ID: 72 mm; length: 1000 mm). The heating area was covered with a heat-resistant glass wool procured from Isolite Ceramic Fiber Sdn. Bhd. Malaysia.
Characterization of the synthesized samples was carried out to classify the type and shape of nanomaterials. Field Emission Scanning Electron Microscopic analysis (FESEM—Hitachi-SU8000, Japan) was used to observe the morphology of the prepared samples. A layer of platinum was coated over the Aluminum stubs by sputtering and the synthesized sample was placed over it for FESEM analysis. Transmission electron microscopic (TEM) analysis was carried out using an Hitachi-HT7700, Japanese microscope at 120 kV. Synthesized samples were mixed with acetone and the mixture was ultrasonicated. After ultrasonication, a drop of the sample was deposited over the copper grid-supported perforated carbon film. The average diameter and particle size distribution of the nanomaterials were calculated using Image-J software. Contact angle measurement (CA) was carried out by KRUSS Goniometer (DSA100). A microscopic slide made of glass (76 × 26 × 1.2 mm) was covered with double-sided adhesive tape and was used to measure the CA between the nanomaterials and water, wherein water drops (4 μL) were placed onto the CNM’s surface that was pasted onto the tape. The average of triplicate measurements for each sample was taken. The oxidation behavior of the prepared nanomaterials was examined by thermal gravimetric analysis (STA-851, Mettler Toledo, Columbus, OH, USA) at the temperature range of 25–800 °C, heating rate of 10 °C/min, and an oxygen flow rate of 20 mL/min. The Raman spectra of the CNMs were obtained, whereby the laser power was kept constant at 100 using an Ar+ laser (514 nm) and it was focused (50× objective) over an area with a size of around 1.5–2.0 μm (Renishaw in Via, UK).
2.6. Response Surface Methodology and Process Optimization
The influence of three sovereign variables,
Fe (Fe%),
Mo (Mo%), and
tr (reaction time), on the two responses, carbon yield (
CY) and contact angle (
CA), were determined. Design-Expert V7.0 was used and Box–Behnken design (BBD) was employed by using the response surface methodological approach (RSM). RSM was used to elucidate the effects of the main and combined input variables on the output responses selected here (Yield and Contact Angle). It is also used to optimize the process by developing models with the subsequent ANOVA analysis. Therefore, 15 experimental runs were proposed by DOE based on BBD with 3 center points. The independent variable ranges studied were Fe% (0%–5%), Mo% (0%–4%), and reaction time (20–60 min), while gas ratios, temperature, and gas type were fixed at an acetylene to hydrogen ratio of 1:4, 650 °C, and acetylene gas, respectively. The complete design matrix confirming the actual experimental design and the responses are illustrated by
Table 1. The adequacy of the developed model was checked using Analysis of variance (ANOVA). The coefficient of determination (R
2) was determined to illustrate the certainty of the suggested model.