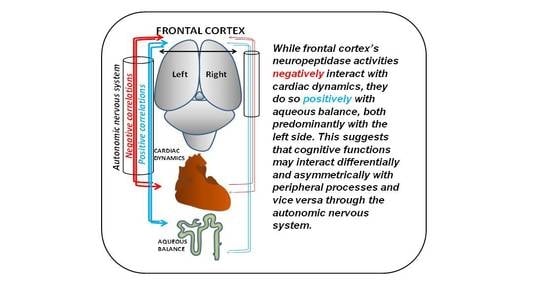
Blood Pressure Correlates Asymmetrically with Neuropeptidase Activities of the Left and Right Frontal Cortices
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Prieto, I.; Villarejo, A.B.; Segarra, A.B.; Banegas, I.; Wangensteen, R.; Martinez-Cañamero, M.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. Brain, heart and kidney correlate for the control of blood pressure and water balance: Role of angiotensinases. Neuroendocrinology 2014, 100, 198–208. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Banegas, I.; Villarejo, A.B.; Wangensteen, R.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. The brain-heart connection: Frontal cortex and left ventricle angiotensinase activities in control and captopril-treated hypertensive rats-a bilateral study. Int. J. Hypertens. 2013, 2013, 156179. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.B.; Prieto-Gomez, I.; Banegas, I.; Martínez-Cañamero, M.; Luna, J.D.; de Gasparo, M.; Ramírez-Sánchez, M. Functional and neurometabolic asymmetry in SHR and WKY rats following vasoactive treatments. Sci. Rep. 2019, 9, 16098. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.M. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress 2004, 7, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V.H. Hemispheric laterality in animals and the effects of early experience. Behav. Brain Sci. 1981, 4, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains. In The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Prieto, I.; Segarra, A.B.; Villarejo, A.B.; de Gasparo, M.; Martínez-Cañamero, M.M.; Ramírez-Sánchez, M. Neuropeptidase activity in the frontal cortex of Wistar-Kyoto and spontaneously hypertensive rats treated with vasoactive drugs: A bilateral study. J. Hypertens. 2019, 37, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, M.; Prieto, I.; Wangensteen, R.; Banegas, I.; Segarra, A.B.; Villarejo, A.B.; Vives, F.; Cobo, J.; de Gasparo, M. The renin-angiotensin system: New insight into old therapies. Curr. Med. Chem. 2013, 20, 1313–1322. [Google Scholar] [CrossRef]
- Chai, S.Y.; Fernando, R.; Peck, G.; Ye, S.Y.; Mendelsohn, F.A.; Jenkins, T.A.; Albiston, A.L. The angiotensin IV/AT4 receptor. Cell. Mol. Life Sci. 2004, 61, 2728–2737. [Google Scholar] [CrossRef]
- Eshima, K.; Hirooka, Y.; Shigematsu, H.; Matsuo, I.; Koike, G.; Sakai, K.; Takeshita, A. Angiotensin in the nucleus tractus solitarii contributes to neurogenic hypertension caused by chronic nitric oxide synthase inhibition. Hypertension 2000, 36, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Priviero, F.B.; Teixeira, C.E.; Claudino, M.A.; De Nucci, G.; Zanesco, A.; Antunes, E. Vascular effects of long-term propranolol administration after chronic nitric oxide blockade. Eur. J. Pharmacol. 2007, 571, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Aretxaga-Maza, G.; Prieto, I.; Luna, J.D.; de Gasparo, M.; Ramírez-Sánchez, M. Diurnal opposite variation between angiotensinase activities in photo-neuro-endocrine tissues of rats. Chronobiol. Int. 2017, 34, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Prieto, I.; Banegas, I.; Segarra, A.B.; Alba, F. Neuropeptidases. Methods Mol. Biol. 2011, 789, 287–294. [Google Scholar] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: London, UK, 1998. [Google Scholar]
- Weiss, L.; Lundgren, Y.; Folkow, B. Effects of prolonged treatment with adrenergic beta-receptor antagonists on blood pressure, cardiovascular design and reactivity in spontaneously hypertensive rats (SHR). Acta Physiol. Scand. 1974, 91, 447–457. [Google Scholar] [CrossRef] [PubMed]
- DeBlois, D.; Tea, B.S.; Than, V.D.; Tremblay, J.; Hamet, P. Smooth muscle apoptosis during vascular regression in spontaneously hypertensive rats. Hypertension 1997, 29, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Slaiby, J.M.; Ricci, M.A.; Gadowski, G.R.; Hendley, E.D.; Pilcher, D.B. Expansion of aortic aneurysms is reduced by propranolol in a hypertensive rat model. J. Vasc. Surg. 1994, 20, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Wittling, W.; Block, A.; Genzel, S.; Schweiger, E. Hemisphere asymmetry in parasympathetic control of the heart. Neuropsychologia 1998, 36, 461–468. [Google Scholar] [CrossRef]
- Diedrich, A.; Porta, A.; Barbic, F.; Brychta, R.J.; Bonizzi, P.; Diedrich, L.; Cerutti, S.; Robertson, D.; Furlan, R. Lateralization of expression of neural sympathetic activity to the vessels and effects of carotid baroreceptor stimulation. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1758–H1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, C.H.; Beig, M.I.; Ianzer, D.; Fontes, M.A.; Nalivaiko, E. Asymmetry in the control of cardiac performance by dorsomedial hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R664–R674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banegas, I.; Prieto, I.; Segarra, A.B.; Durán, R.; Vives, F.; Alba, F.; Luna, J.D.; de Gasparo, M.; Wangensteen, R.; Ruiz-Bailen, M.; et al. Blood pressure increased dramatically in hypertensive rats after left hemisphere lesions with 6-hydroxydopamine. Neurosci. Lett. 2011, 500, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.; Kaneda, N.; Ishii, A.; Mogi, M.; Harada, M.; Nagatsu, T. Right-left asymmetry of tyrosine hydroxylase in rat median eminence: Influence of arterial baroreflex nerves. Brain Res. 1990, 523, 195–198. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Gratton, A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 1999, 19, 2834–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseger, T.A.; van Bueren, N.E.R.; Kenemans, J.L.; Gevirtz, R.; Arns, M. A frontal-vagal network theory for major depressive disorder: Implications for optimizing neuromodulation techniques. Brain Stimul. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.A.; Glick, S.D.; Meibach, R.C. Sexually dimorphic brain and behavioural asymmetries in the neonatal rat. Proc. Natl. Acad. Sci. USA 1981, 78, 1958–1961. [Google Scholar] [CrossRef] [Green Version]
- Pilli, V.K.; Jeong, J.W.; Konka, P.; Kumar, A.; Chugani, H.T.; Juhász, C. Objective PET study of glucose metabolism asymmetries in children with epilepsy: Implications for normal brain development. Hum. Brain Mapp. 2019, 40, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Hooghiemstra, A.M.; Bertens, A.S.; Leeuwis, A.E.; Bron, E.E.; Bots, M.L.; Brunner-La Rocca, H.P.; de Craen, A.J.M.; van der Geest, R.J.; Greving, J.P.; Kappelle, L.J.; et al. Heart-brain connection consortium, the missing link in the pathophysiology of vascular cognitive impairment: Design of the heart-brain study. Cerebrovasc. Dis. Extra 2017, 7, 140–152. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef]
- Schrier, R.W.; Berl, T. Mechanism of the antidiuretic effect associated with interruption of parasympathetic pathways. J. Clin. Investig. 1972, 51, 2613–2620. [Google Scholar] [CrossRef]
- Prieto, I.; Segarra, A.B.; Martinez-Canamero, M.; de Gasparo, M.; Zorad, S.; Ramirez-Sanchez, M. Bidirectional asymmetry in the neurovisceral communication for the cardiovascular control: New insights. Endocr. Regul. 2017, 51, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Banegas, I.; Prieto, I.; Segarra, A.B.; Martínez-Cañamero, M.; de Gasparo, M.; Ramírez-Sánchez, M. Angiotensin II, dopamine and nitric oxide. An asymmetrical neurovisceral interaction between brain and plasma to regulate blood pressure. Aims Neurosci. 2019, 6, 116–127. [Google Scholar] [CrossRef]
- Smith, R.; Thayer, J.F.; Khalsa, S.S.; Lane, R.D. The hierarchical basis of neurovisceral integration. Neurosci. Biobehav. Rev. 2017, 75, 274–296. [Google Scholar] [CrossRef] [PubMed]
- Richter, F.; García, A.M.; Rodriguez Arriagada, N.; Yoris, A.; Birba, A.; Huepe, D.; Zimmer, H.; Ibáñez, A.; Sedeño, L. Behavioral and neurophysiological signatures of interoceptive enhancements following vagus nerve stimulation. In Human Brain Mapping; Wiley Periodicals LLC: New York, NY, USA, 2020; Epub ahead of print. [Google Scholar]
- Diz, D.I.; Ferrario, C.M. Bidirectional transport of angiotensin II binding sites in the vagus nerve. Hypertension 1988, 11, I139–I143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.O.; Coleman, M.P. KIF1A mediates axonal transport of BACE1 and identification of independently moving cargoes in living SCG neurons. Traffic 2016, 17, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Sudilovsky, A.; Turnbull, B.; Croog, S.H.; Crook, T. Angiotensin converting enzyme and memory: Preclinical and clinical data. Int. J. Neurol. 1987, 21–22, 145–162. [Google Scholar]
- Craig, A.D. Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn. Sci. 2005, 9, 566–571. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segarra, A.B.; Prieto, I.; Banegas, I.; Martínez-Cañamero, M.; de Gasparo, M.; Ramírez-Sánchez, M. Blood Pressure Correlates Asymmetrically with Neuropeptidase Activities of the Left and Right Frontal Cortices. Symmetry 2021, 13, 105. https://doi.org/10.3390/sym13010105
Segarra AB, Prieto I, Banegas I, Martínez-Cañamero M, de Gasparo M, Ramírez-Sánchez M. Blood Pressure Correlates Asymmetrically with Neuropeptidase Activities of the Left and Right Frontal Cortices. Symmetry. 2021; 13(1):105. https://doi.org/10.3390/sym13010105
Chicago/Turabian StyleSegarra, Ana Belén, Isabel Prieto, Inmaculada Banegas, Magdalena Martínez-Cañamero, Marc de Gasparo, and Manuel Ramírez-Sánchez. 2021. "Blood Pressure Correlates Asymmetrically with Neuropeptidase Activities of the Left and Right Frontal Cortices" Symmetry 13, no. 1: 105. https://doi.org/10.3390/sym13010105
APA StyleSegarra, A. B., Prieto, I., Banegas, I., Martínez-Cañamero, M., de Gasparo, M., & Ramírez-Sánchez, M. (2021). Blood Pressure Correlates Asymmetrically with Neuropeptidase Activities of the Left and Right Frontal Cortices. Symmetry, 13(1), 105. https://doi.org/10.3390/sym13010105