Metasomatic Reactions between Archean Dunite and Trondhjemite at the Seqi Olivine Mine in Greenland
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
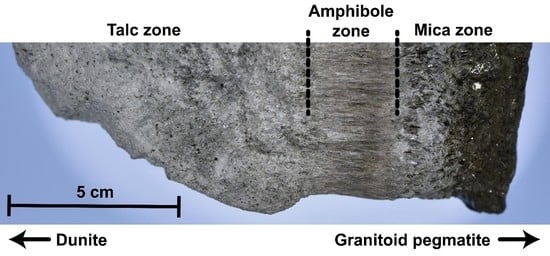
3.1. Samples and Petrography
3.2. Methods
3.2.1. Bulk-Rock Geochemical Data
3.2.2. Electron Microprobe Analysis
3.2.3. Powder X-ray Diffraction
3.2.4. Triple Oxygen Isotope Analysis
3.2.5. Zircon U–Pb Isotope Analysis
4. Results
4.1. Bulk-Rock Geochemical Data
4.2. Electron Microprobe Analysis
4.3. Powder X-ray Diffraction
4.4. Triple Oxygen Isotope Analysis
4.5. Zircon U-Pb Isotope Analysis
5. Discussion
5.1. Pressure-Temperature-Time Constrains of Talc Fomation
5.2. Oxygen Isotope Constraints on the Origin and Temperature of Metasomatic Fluids
5.3. Metasomatic Element Mobility
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nutman, A.P.; McGregor, V.R.; Friend, C.R.; Bennett, V.C.; Kinny, P.D. The Itsaq gneiss complex of southern West Greenland; the world’s most extensive record of early crustal evolution (3900–3600 Ma). Precambrian Res. 1996, 78, 1–39. [Google Scholar] [CrossRef]
- Nutman, A.P.; Friend, C.R.; Hiess, J. Setting of the ∼2560 Ma Qôrqut Granite complex in the Archean crustal evolution of southern west Greenland. Am. J. Sci. 2010, 310, 1081–1114. [Google Scholar] [CrossRef] [Green Version]
- Szilas, K. A geochemical overview of mid-Archaean metavolcanic rocks from southwest Greenland. Geosciences 2018, 8, 266. [Google Scholar] [CrossRef] [Green Version]
- Windley, B.F.; Garde, A.A. Arc-generated blocks with crustal sections in the North Atlantic craton of West Greenland: Crustal growth in the Archean with modern analogues. Earth-Sci. Rev. 2009, 93, 1–30. [Google Scholar] [CrossRef]
- Szilas, K.; van Hinsberg, V.; McDonald, I.; Næraa, T.; Rollinson, H.; Adetunji, J.; Bird, D. Highly refractory Archaean peridotite cumulates: Petrology and geochemistry of the Seqi Ultramafic Complex, SW Greenland. Geosci. Front. 2018, 9, 689–9714. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, T.; Pearson, D.G.; Szilas, K.; Morishita, T. Implications for the origins of Eoarchean ultramafic rocks of the North Atlantic Craton: A study of the Tussaap Ultramafic complex, Itsaq Gneiss complex, southern West Greenland. Contrib. Mineral. Petrol. 2019, 174, 96. [Google Scholar] [CrossRef]
- Keulen, N.; Schumacher, J.C.; Næraa, T.; Kokfelt, T.F.; Scherstén, A.; Szilas, K.; van Hinsberg, V.J.; Schlatter, D.M.; Windley, B.F. Meso-and Neoarchaean geological history of the Bjørnesund and Ravns Storø Supracrustal Belts, southern West Greenland: Settings for gold enrichment and corundum formation. Precambrian Res. 2014, 254, 36–58. [Google Scholar] [CrossRef]
- Yakymchuk, C.; Szilas, K. Corundum formation by metasomatic reactions in Archean metapelite, SW Greenland: Exploration vectors for ruby deposits within high-grade greenstone belts. Geosci. Front. 2018, 9, 727–9749. [Google Scholar] [CrossRef]
- Szilas, K.; Kelemen, P.B.; Bernstein, S. Peridotite enclaves hosted by Mesoarchaean TTG-suite orthogneisses in the Fiskefjord region of southern West Greenland. GeoResJ 2015, 7, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Friend, C.R.; Nutman, A.P. New pieces to the Archaean terrane jigsaw puzzle in the Nuuk region, southern West Greenland: Steps in transforming a simple insight into a complex regional tectonothermal model. J. Geol. Soc. 2005, 162, 147–162. [Google Scholar] [CrossRef]
- Garde, A.A. Accretion and evolution of an Archaean high-grade grey gneiss—Amphibolite complex: The Fiskefjord area, southern West Greenland. Geol. Greenl. Surv. Bull. 1997, 177, 115. [Google Scholar]
- Garde, A.A.; Friend, C.R.; Nutman, A.P.; Marker, M. Rapid maturation and stabilisation of middle Archaean continental crust: The Akia terrane, southern West Greenland. Bull. Geol. Soc. Den. 2000, 47, 1–27. [Google Scholar]
- Gardiner, N.J.; Kirkland, C.L.; Hollis, J.; Szilas, K.; Steenfelt, A.; Yakymchuk, C.; Heide-Jørgensen, H. Building Mesoarchaean crust upon Eoarchaean roots: The Akia Terrane, West Greenland. Contrib. Mineral. Petrol. 2019, 174, 20. [Google Scholar] [CrossRef] [Green Version]
- Garde, A.A. A mid-Archaean island arc complex in the eastern Akia terrane, Godthåbsfjord, southern West Greenland. J. Geol. Soc. 2007, 164, 565–579. [Google Scholar] [CrossRef]
- Szilas, K.; Tusch, J.; Hoffmann, J.E.; Garde, A.A.; Münker, C. Hafnium isotope constraints on the origin of Mesoarchaean andesites in southern West Greenland, North Atlantic craton. Geol. Soc. Lond. Spec. Publ. 2017, 449, 19–38. [Google Scholar] [CrossRef]
- Yakymchuk, C.; Kirkland, C.; Hollis, J.; Kendrick, J.; Gardiner, N.; Szilas, K. Mesoarchean partial melting of mafic crust and tonalite production during high-T–low-P stagnant tectonism, Akia Terrane, West Greenland. Precambrian Res. 2019, in press. [Google Scholar] [CrossRef]
- Washington State University GeoAnalytical Lab. Technical Notes Describing Sample Preparation, Analytical Procedure, Precision, and Accuracy of XRF and ICP-MS Analysis. Website Visited on 29 November 2019. Available online: https://environment.wsu.edu/facilities/geoanalytical-lab/technical-notes/ (accessed on 20 January 2020).
- Waight, T.E.; Tørnqvist, J.B. Sr isotope zoning in plagioclase from andesites at Cabo De Gata, Spain: Evidence for shallow and deep contamination. Lithos 2018, 308, 159–167. [Google Scholar] [CrossRef]
- Pack, A.; Tanaka, R.; Hering, M.; Sengupta, S.; Peters, S.; Nakamura, E. The oxygen isotope composition of San Carlos olivine on the VSMOW2-SLAP2 scale. Rapid Commun. Mass Spectrom. 2016, 30, 1495–1504. [Google Scholar] [CrossRef]
- Pack, A.; Herwartz, D. The triple oxygen isotope composition of the Earth mantle and understanding Δ17O variations in terrestrial rocks and minerals. Earth Planet. Sci. Lett. 2014, 390, 138–145. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Wostbrock, J.A.G.; Pack, A. Mass-dependent triple oxygen isotope variations in terrestrial materials. Geochem. Perspect. Lett. 2018, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Valley, J.W.; Kitchen, N.; Kohn, M.J.; Niendorf, C.R.; Spicuzza, M.J. UWG-2, a garnet standard for oxygen isotope ratios: Strategies for high precision and accuracy with laser heating. Geochim. Cosmochim. Acta 1995, 59, 5223–5231. [Google Scholar] [CrossRef]
- Stern, R.A.; Bodorkos, S.; Kamo, S.L.; Hickman, A.H.; Corfu, F. Measurement of SIMS instrumental mass fractionation of Pb isotopes during zircon dating. Geostand. Geoanal. Res. 2009, 33, 145–168. [Google Scholar] [CrossRef]
- Wiedenbeck, M.A.; Alle, P.; Corfu, F.; Griffin, W.L.; Meier, M.; Oberli, F.V.; Quadt, A.V.; Roddick, J.C.; Spiegel, W. Three natural zircon standards for U–Th–Pb, Lu–Hf, trace element and REE analyses. Geostand. Newsl. 1995, 19, 1–23. [Google Scholar] [CrossRef]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Sláma, J.; Košler, J.; Condon, D.J.; Crowley, J.L.; Gerdes, A.; Hanchar, J.M.; Horstwood, M.S.; Morris, G.A.; Nasdala, L.; Norberg, N.; et al. Plešovice zircon—A new natural reference material for U–Pb and Hf isotopic microanalysis. Chem. Geol. 2008, 249, 1–35. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Bindeman, I. Oxygen isotopes in mantle and crustal magmas as revealed by single crystal analysis. Rev. Mineral. Geochem. 2008, 69, 445–478. [Google Scholar] [CrossRef]
- Mattey, D.; Lowry, D.; Macpherson, C. Oxygen isotope composition of mantle peridotite. Earth Planet. Sci. Lett. 1994, 128, 231–241. [Google Scholar] [CrossRef]
- Guotana, J.; Morishita, T.; Yamaguchi, R.; Nishio, I.; Tamura, A.; Tani, K.; Harigane, Y.; Szilas, K.; Pearson, D. Contrasting textural and chemical signatures of chromitites in the Mesoarchaean Ulamertoq peridotite body, southern West Greenland. Geosciences 2018, 8, 328. [Google Scholar] [CrossRef] [Green Version]
- Nishio, I.; Morishita, T.; Szilas, K.; Pearson, G.; Tani, K.I.; Tamura, A.; Harigane, Y.; Guotana, J.M. Titanian Clinohumite-Bearing Peridotite from the Ulamertoq Ultramafic Body in the 3.0 Ga Akia Terrane of Southern West Greenland. Geosciences 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, C.L.; Yakymchuk, C.; Szilas, K.; Evans, N.; Hollis, J.; McDonald, B.; Gardiner, N.J. Apatite: A U-Pb thermochronometer or geochronometer? Lithos 2018, 318, 143–157. [Google Scholar] [CrossRef]
- Cochrane, R.; Spikings, R.A.; Chew, D.; Wotzlaw, J.F.; Chiaradia, M.; Tyrrell, S.; Schaltegger, U.; Van der Lelij, R. High temperature (>350 °C) thermochronology and mechanisms of Pb loss in apatite. Geochim. Cosmochim. Acta 2014, 127, 39–56. [Google Scholar] [CrossRef]
- Schoene, B.; Bowring, S.A. Determining accurate temperature–time paths from U–Pb thermochronology: An example from the Kaapvaal craton, southern Africa. Geochim. Cosmochim. Acta 2007, 71, 165–185. [Google Scholar] [CrossRef]
- Nutman, A.P.; Rivers, T.; Longstaffe, F.; Park, J.F. The Ataneq fault and mid-Proterozoic retrograde metamorphism of early Archaean tonalites of the Isukasia area, southern West Greenland: Reactions, fluid compositions and implications for regional studies. In Fluid Movements—Element Transport and the Composition of the Deep Crust; Springer: Dordrecht, The Netherlands, 1989; pp. 151–170. [Google Scholar]
- Friend, C.R.; Nutman, A.P.; Baadsgaard, H.; Kinny, P.D.; McGregor, V.R. Timing of late Archaean terrane assembly, crustal thickening and granite emplacement in the Nuuk region, southern West Greenland. Earth Planet. Sci. Lett. 1996, 142, 353–365. [Google Scholar] [CrossRef]
- Nutman, A.P.; Christiansen, O.; Friend, C.R. 2635 Ma amphibolite facies gold mineralisation near a terrane boundary (suture?) on Storø, Nuuk region, southern West Greenland. Precambrian Res. 2007, 159, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Scherstén, A.; Szilas, K.; Creaser, R.A.; Næraa, T.; van Gool, J.A.; Østergaard, C. Re–Os and U–Pb constraints on gold mineralisation events in the Meso- to Neoarchaean Storø greenstone belt, Storø, southern West Greenland. Precambrian Res. 2012, 200, 149–162. [Google Scholar] [CrossRef]
- Szilas, K.; van Hinsberg, V.J.; McDonald, I.; Morishita, T.; Pearson, D.G. Highly depleted peridotites within Mesoarchaean orthogneiss at the Seqi Olivine Mine, SW Greenland—Potential implications for the formation of cratonic keels. Goldschmidt Conf. Abstr. Yokohama 2016, 3009, 1. [Google Scholar]
- Trommsdorff, V.; Connolly, J.A. The ultramafic contact aureole about the Bregaglia (Bergell) tonalite: Isograds and a thermal model. Schweiz. Mineral. Und Petrogr. Mitt. 1996, 76, 537–547. [Google Scholar]
- Richter, R.; Hoernes, S. The application of the increment method in comparison with experimentally derived and calculated O-isotope fractionations. Chem. Erde 1988, 48, 1–18. [Google Scholar]
- Zheng, Y.F. Calculation of oxygen isotope fractionation in hydroxyl-bearing silicates. Earth Planet. Sci. Lett. 1993, 120, 247–263. [Google Scholar] [CrossRef]
- Matsuhisa, Y.; Goldsmith, J.R.; Clayton, R.N. Oxygen isotopic fractionation in the system quartz-albite-anorthite-water. Geochim. Cosmochim. Acta 1979, 43, 1131–1140. [Google Scholar] [CrossRef]
- Bottinga, Y.; Javoy, M. Comments on oxygen isotope geothermometry. Earth Planet. Sci. Lett. 1973, 20, 250–265. [Google Scholar] [CrossRef]
- Peters, S.T.M.; Szilas, K.; Pack, A.; Sengupta, S.; Kirkland, C.; Gabe-Schönberg, D. Oxygen isotope compositions of >2.7 Ga aqueous fluids recorded in metamorphic peridotites from southeast Greenland. Earth Planet. Sci. Lett. 2020. in review. [Google Scholar]
- Grant, J.A. The isocon diagram; a simple solution to Gresens’ equation for metasomatic alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- López-Moro, F.J. EASYGRESGRANT—A Microsoft Excel spreadsheet to quantify volume changes and to perform mass-balance modeling in metasomatic systems. Comput. Geosci. 2012, 39, 191–196. [Google Scholar] [CrossRef]











| Formula | Sites | Mineral |
|---|---|---|
| Na0.01(Ca0.04Mg5.36Mn0.1Fe1.42Ni0.03)Si8.01O22(OH)2 | Σ A(0.01), M(6.95), T(8.01) | Anthophyllite |
| Σ M(3.15) | Chromite | |
| (K0.72Na0.05Ca0.02)(Mg2.67Fe0.25Al0.02Cr0.02Ni0.02Ti0.01)(Si3.09Al0.91)O10(OH)2 | Σ A(0.79), M(2.99), T(4) | Phlogopite |
| Σ M(2.94), T(4.03) | Talc |
| Formula | Sites | Mineral |
|---|---|---|
| Na0.01(Ca0.05Mg5.36Fe1.38Mn0.08Ni0.03Al0.01)Si8.05O22(OH)2 | Σ A(0.01), M(6.85), T(8.05) | Anthophyllite |
| Σ M(3.18) | Chromite | |
| (K0.82Na0.05Ca0.01)(Mg2.65Fe0.26Ni0.02Al0.02Cr0.03Ti0.01)(Al0.94Si3.06)O10(OH)2 | Σ A(0.88), M(2.99), T(4) | Phlogopite |
| (Mg2.81Fe0.1Ni0.03)Si4.01O10(OH)2 | Σ M(2.94), T(4.01) | Talc |
| Formula | Sites | Mineral |
|---|---|---|
| (K0.84Na0.05Ca0.01)(Mg2.66Fe0.26Ni0.02Al0.03Ti0.01)(Al0.94Si3.06)O10(OH)2 | Σ A(0.9), M(2.98), T(4) | Phlogopite |
| (Na0.01Mg2.85Fe0.11Ni0.02Al0.01)Si3.99O10(OH)2 | Σ M(3), T(4) | Talc |
| Formula | Sites | Mineral |
|---|---|---|
| Σ A(1.03), T(3.98) | Albite | |
| (K0.93 Na0.01)(Mg1.45Fe1.07Al0.20Ti0.12Mn0.01)(Si2.88Al1.12)O10(OH)2 | Σ A(0.94), M(2.85), T(4) | Biotite |
| K0.01(Na0.05Ca1.89Mg3.87Fe1.04Al0.1)Si8O22(OH)2 | Σ A(0.01), M(6.95), T(8) | Tremolite |
| SiO2 | Quartz |
| Talc Zone | Formula | wt.% |
|---|---|---|
| Talc | Mg2.9Fe0.1Si4O10(OH)2 | 93.7 |
| Anthophyllite | Mg5.76Fe1.24Si8O22(OH)2 * | 0.5 |
| Phlogopite | KMg2.76Fe0.24AlSi3O10(OH)2 | 5.8 |
| Amphibole Zone | Formula | wt.% |
| Talc | Mg2.8Fe0.2Si4O10(OH)2 | 1 |
| Anthophyllite | Mg5.76Fe1.24Si8O22(OH)2 | 92 |
| Phlogopite | KMg2.76Fe0.24AlSi3O10(OH)2 | 7 |
| Chromite | Cr2FeO4 * | <0.1 |
| Mica Zone | Formula | wt.% |
| Talc | Mg2.9Fe0.1Si4O10(OH)2 | 4 |
| Phlogopite | KMg2.93Fe0.07Al1.19Si2.81O10(OH)2 | 61.3 |
| Tremolite | Ca1.84Fe0.08Mg5Si8O22(OH)2 | 34.5 |
| Chlorite | Mg3Fe3Al1.39Si2.61O10(OH)8 | 0.2 |
| Sample | δ17O [‰] | δ18O [‰] | Δ’17O0.528 [ppm] |
|---|---|---|---|
| 186452 Dunite | 2.71 | 5.19 | −27 |
| 186478 Dunite | 2.66 | 5.11 | −43 |
| 208001 Talc zone | 3.34 | 6.40 | −37 |
| 208002 Amphibole zone | 3.21 | 6.17 | −40 |
| 208003 Mica zone | 2.94 | 5.65 | −33 |
| 186453 Trondhjemite | 4.57 | 8.76 | −43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whyatt, L.; Peters, S.; Pack, A.; Kirkland, C.L.; Balic-Zunic, T.; Szilas, K. Metasomatic Reactions between Archean Dunite and Trondhjemite at the Seqi Olivine Mine in Greenland. Minerals 2020, 10, 85. https://doi.org/10.3390/min10010085
Whyatt L, Peters S, Pack A, Kirkland CL, Balic-Zunic T, Szilas K. Metasomatic Reactions between Archean Dunite and Trondhjemite at the Seqi Olivine Mine in Greenland. Minerals. 2020; 10(1):85. https://doi.org/10.3390/min10010085
Chicago/Turabian StyleWhyatt, Laura, Stefan Peters, Andreas Pack, Christopher L. Kirkland, Tonci Balic-Zunic, and Kristoffer Szilas. 2020. "Metasomatic Reactions between Archean Dunite and Trondhjemite at the Seqi Olivine Mine in Greenland" Minerals 10, no. 1: 85. https://doi.org/10.3390/min10010085
APA StyleWhyatt, L., Peters, S., Pack, A., Kirkland, C. L., Balic-Zunic, T., & Szilas, K. (2020). Metasomatic Reactions between Archean Dunite and Trondhjemite at the Seqi Olivine Mine in Greenland. Minerals, 10(1), 85. https://doi.org/10.3390/min10010085