3.2. Flowsheet Development
The development of suitable flowsheets and optimised reagent regimes for the processing of ores A and B was based on the ores’ mineralogical characteristics. Preliminary reagent scouting tests showed that Flotinor 7243 and Arizona Sylfat FA2, both fatty acid collectors, produced superior metallurgical results for the processing of ore A and B, respectively. The results were not presented not only for the brevity of the manuscript but also because this paper focussed only on the role of gangue mineralogy on flowsheet development for fluorite processing. Preliminary flotation testwork also showed that six cleaning stages were required to produce an acid-grade CaF2 product from both ores.
3.2.1. Flowsheet for Ore A
The mineralogical and chemical analysis results depicted in
Table 2 and
Figure 1 indicate that for ore A to be upgraded, quartz and calcite minerals are to be separated from the fluorite during flotation. Tannins have been shown to be strong calcite depressants [
6,
7], while sodium silicate is reported to be an effective silica depressant [
8,
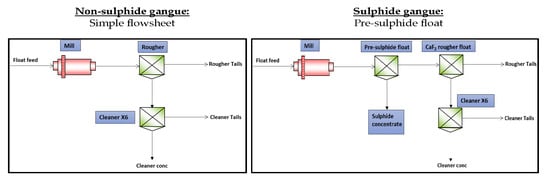
9]. In terms of liberation characteristics at a grind of 80% passing 150 µm, 90% of the fluorite grains were fully liberated, with less than 15% of fluorite grains showing an intimate association with calcite and other silicate gangue minerals. Therefore, a grind of 80% passing 150 µm was assumed suitable to achieve adequate liberation of selective fluorite flotation. A simple flotation flowsheet, shown in
Figure 2, was proposed for processing ore A. Flotation times were as follows: 4 min for the rougher; 4 min for cleaners 1 and 2; 3 min for cleaners 3 and 4; 2 min for cleaners 5 and 6.
3.2.2. Flowsheet for Ore B
The beneficiation of ore B required the depression of pyrite, calcite and dolomite. Similar to ore A, tannins and sodium silicate were used as calcite and silica depressants [
6,
7,
8,
9]. Lignin sulphonate is reported to be an effective pyrite depressant [
10]. In terms of liberation characteristics at a grind of 80% passing 150 µm, 25% of fluorite grains were fully liberated with 75% of fluorite grains reporting as middlings and 42% fluorite being associated with major gangue. Because of the poor degree of liberation, ore B might require concentrate regrinding to liberate the fluorite grains as opposed to ore A. To determine the optimum grind for ore B, the effect of grind on the grade–recovery relationship in rougher flotation was investigated and the results are shown in
Table 3.
Table 3 shows that the rougher CaF
2 recoveries for P
80 grinds of 125 µm and 106 µm were similar at 86%, while for P
80 of 90 µm and 75 µm the rougher CaF
2 recoveries were similar at 89%. The rougher CaF
2 grades decreased in the following order: P
80 = 106 µm > P
80 = 90 µm > P
80 = 125 µm > P
80 = 75 µm. Because it produced the highest CaF
2 grade at reasonably high CaF
2 recoveries, the 80% passing 106 µm grind was selected as the optimum grind for further flotation testwork with a view to improving metallurgical performance.
Different process routes were considered for the removal of the pyritic gangue in ore B. The first route was through the selective depression of the pyritic gangue using the lignin sulphonate depressant, employing the simple flowsheet shown in
Figure 2. The second route was an upfront removal of the pyritic gangue using a pre-sulphide flotation cell as shown in
Figure 3. The second route used NaHS as a sulphide activator [
2,
11] at a low dosage of 15 g/t and potassium amyl xanthate (PAX) as a sulphide collector [
12] in a pre-sulphide flotation cell. A higher NaHS dosage would be detrimental as it would depress the pyrite. NaHS functions by either cleaning or sulphidising the pyrite surface and hence improving the pyrite floatability. Flotation times were as follows: 6 min for the pre-sulphide rougher; 4 min for the rougher; 4 min for cleaners 1 and 2; 3 min for cleaners 3 and 4; 2 min for cleaners 5 and 6. The metallurgical performance of the two process routes was evaluated and the results are presented in
Table 4.
Table 4 shows, that although both process routes produced an acid-grade CaF
2 product, the pre-sulphide flotation route produced 50% less sulphur content compared to the selective depression route. The pre-sulphide flotation route produced higher CaF
2 recovery and grade than the selective depression route. It is clear that the pre-sulphide flotation route was the most effective means of removing the pyritic gangue from the fluorite ore and hence the flowsheet in
Figure 3 was used for further optimisation tests.
Although the pre-sulphide flotation route was successful in reducing the pyritic gangue reporting to the CaF2 concentrate, the sulphur content was still higher than the market specification of at most 0.03%. Therefore, optimisation tests were conducted to reduce the sulphur content in the CaF2 concentrate.
3.3. Effect of Pulp Temperature
Figure 4 shows the effect of pulp temperature on the CaF
2 grade–recovery relationship for ore A using the Flotinor 7243 collector at 300 g/t dosage. The objective was to evaluate the affinity and the subsequent efficiency of the collector towards different minerals within the ore as a function of pulp temperature.
Figure 4 shows that as the pulp temperature increases, the CaF
2 recovery increases, while the CaF
2 grade decreases. It is clear from
Figure 3 that although the CaF
2 recovery was low around 56%, the product grade met the market specification of 97% CaF
2 at 20 °C. However, it was not possible to produce the acid-grade concentrate at 40 °C. In order to investigate whether the observed results were ore-specific or not, the effect of pulp temperature was also investigated for ore B using a different fatty acid collector, Sylfat FA2 at 300 g/t dosage, and the results are presented in
Figure 5.
The results in
Figure 5 show that an acid-grade concentrate with a grade of 97% CaF
2 can be attained at 75% and 85% recoveries using the Sylfat FA2 collector at 20 °C and 40 °C, respectively. A further increase of the pulp temperature to 60 °C resulted in a significant increase in CaF
2 recovery, but at the expense of CaF
2 grade. It was therefore not possible to produce an acid-grade concentrate at 60 °C using the Sylfat FA2 collector.
It has been observed from
Figure 4 and
Figure 5, that as the pulp temperature increases, the CaF
2 recovery increases, while the CaF
2 grade decreases for both ores. Thus, the results are not ore-specific. Similar observations were made by [
4], who showed that better fluorite selectivity in carbonate-fluorite ores is achievable at low temperatures. The same finding was made by [
3] in their investigation on a fluorite ore from China using sodium naphthenate and salted copper sulphate as collector and depressant of phosphate gangue minerals, respectively. The results presented in both
Figure 4 and
Figure 5 suggest that operating at lower temperatures would be more favourable to produce an acid-grade fluorite product. The results therefore show that both Flotinor 7243 and Sylfat FA2 can be effectively used as collectors in the flotation of ore A and B, respectively, to produce acid-grade CaF
2 concentrate at low pulp temperatures. Besides the benefit of increased selectivity, operating at low pulp temperatures would result in a significant reduction in energy costs in fluorite flotation. This is especially true for most fluorite processing plants located in northern China, which are characterised by long cold winters. In such plants, energy consumption is high due to heating of the ore slurry in flotation circuits for efficient separation. The use of the Flotinor 7243 and Sylfat FA2 collectors therefore eliminates the need for high-temperature pulp treatment, and thus a significant reduction in energy costs.
To understand the reason behind the loss of selectivity as pulp temperature increases for ore B, the behaviour of gangue minerals as a function of pulp temperature was investigated, and the results are presented in
Table 5.
Table 5 shows that the calcite contamination increased with increasing pulp temperature. The increase in CaF
2 recoveries with increasing pulp temperature could be due to an increase in the hydrophobicity and subsequent floatability of CaF
2 as pulp temperature increases. This could be a result of improved dispersion of the collector throughout the pulp at high pulp temperatures.
Improved collector dispersion and increased rate of mineral–collector interactions at high temperatures may promote the unselective adsorption of the fatty acid collector onto the gangue minerals. This renders the gangue minerals floatable, and hence the observed increase in gangue dilution of the CaF
2 concentrate as pulp temperature increases. A similar finding was made by [
13] in ilmenite flotation, where they observed the improved floatability of feldspar and biotite gangue minerals as pulp temperature was increased, resulting in reduced low-grade ilmenite concentrates.
3.4. Effect of Fluorite Activation on Fluorite Flotation
The addition of sodium fluoride, NaF, as a fluorite activator on the flotation performance of both ores A and B was investigated in the rougher flotation cell and the results are shown in
Table 6. It is clear from
Table 6 that increasing NaF dosage from 0 to 500 g/t resulted in a slight increase in the recovery of CaF
2 but a significant increase in the concentrate grade for ore A. In fact, the concentrate grade was increased by more than 12%. The significant increase in CaF
2 grade was accompanied by around a 50% decrease in silica and calcite gangue content. However, a further 50% increase in the NaF dosage resulted in a 3% drop in the CaF
2 grade. This was clearly due to the increase in the recovery of gangue at high NaF dosages.
It is also clear from
Table 6 that CaF
2 recovery dropped by almost 50% when NaF was added to the roughers for ore B. Furthermore, gangue content increased with the addition of NaF. This shows that the use of NaF at 500 g/t dosage had a detrimental effect on fluorite recovery from ore B.
The increase in the CaF
2 grade with increasing NaF dosage observed for ore A is consistent with the findings made by [
4], who concluded that NaF improved fluorite selectivity in the rougher cells. The authors [
4] proposed that the NaF and collector interact, forming fluorine-bearing associates which have a higher affinity for fluorite than gangue minerals, e.g., carbonates and sulphates. They also suggested that fluorite activation is a result of the restructuring of the fluorite surface layer under the influence of the excess F
− ions generated from the NaF addition. Both mechanisms should result in increased fluorite recovery and selectivity. However, the results presented in
Table 6 suggest that there was an optimum dosage (500 g/t NaF) at which the CaF
2 grade was maximum and gangue contamination was minimum for ore A. The results in
Table 6 also indicate that NaF depresses the gangue at low dosages. This is evidenced by the drop in the gangue content, coupled with a significant increase in the CaF
2 product grade by the addition of 500 g/t NaF. It can be therefore concluded that NaF functions both as a fluorite activator and gangue depressant at low dosages.
For ore B, the CaF2 grade just dropped by the addition of 500 g/t of NaF. The results seem to suggest that the 500 g/t NaF dosage was already higher than the optimum NaF dosage. It is therefore recommended that lower NaF dosages be investigated to establish the optimum NaF dosage that would give the best metallurgical benefits in terms of CaF2 grade and recovery for ore B.
At high NaF dosages, the excess fluorine-bearing associates formed from the interaction between the excess NaF and collector may unselectively adsorb onto the gangue minerals. Thus, the gangue minerals become floatable, hence the observed increase in silica and calcite contamination. Neutralisation or passivation of the Ca sites on the fluorite mineral surface by excess F− may also occur, such that there are no longer any Ca sites available on the fluorite mineral surface to bond with the collector on the fluorite surface, hence the loss in CaF2 recovery at high NaF dosages.
3.5. Effect of Sodium Silicate Dosage for Ore A
The effect of adding sodium silicate as a silicate depressant in an attempt to reduce silica content to below 1% in the concentrate was investigated in the rougher flotation cell and the results are shown in
Table 7. It is clear from
Table 7 that only a slight improvement in silica depression was achieved by increasing sodium silicate dosage from 0 to 310 g/t. A further increase in the sodium silicate dosage to 390 g/t resulted in the targeted silica contamination of the less than 1% in the concentrate. However, this this was accompanied by significant loss in CaF
2 recovery. The CaF
2 recovery loss at a high sodium silicate dosage could be attributed to both reduced mass pull and the depression of the fluorite grains that are associated with silica gangue minerals, as revealed by the mineralogical analysis as shown in
Table 8. The lower sodium silicate dosage was therefore not strong enough to depress the silica associated with the fluorite particles. Mineral association data are derived from shared boundaries amongst the identified mineral grains, therefore the higher the associated percentage is, the greater the degree of boundary-sharing between mineral species. In
Table 8, it is clear that there exists some fluorite association with silicate minerals such as calcite, dolomite, pyroxene, K-feldspar and quartz, which ultimately resulted in CaF
2 recovery loss when the depressant dosage was increased. This result indicates that there is an opportunity to explore other silicate depressants in an attempt to maximise the CaF
2 recovery to the product.
3.6. Effect of Concentrate Regrind for Ore B
The results so far have demonstrated that it is possible to produce an acid-grade product with at least 96% CaF
2. However, the S content in the CaF
2 concentrate was well above the market specification of 0.03%. The high S content could be attributed to the association of fluorite grains with sulphide minerals as shown from the mineralogical analysis of the CaF
2 concentrate presented in
Table 9.
Table 9 shows that 24% of the sulphides were associated with fluorite grains and hence the effect of concentrate regrinding on the sulphur content in the CaF
2 concentrate was investigated and the results are shown in
Figure 6 and
Table 10.
To avoid grinding finer than the market’s product PSD specification of 80% passing 65 µm, a milling curve was constructed, which showed that the regrind time of 8 min produced a product with a PSD coarser than a P
80 of 65 µm. The milling curve showed that a regrind time of 1 min was required to achieve a P
80 of 90 µm. Two cleaner concentrates were therefore independently ground for 1 and 2 min.
Figure 6 shows that concentrate regrinding reduced both the CaF
2 recoveries and grades. The drop in CaF
2 recoveries could be due to the slower floating finer particles generated during the concentrate regrind. The drop in the grade of the CaF
2 concentrate grade could be due to both the increased liberation of floatable gangue, such as calcite and entrainment of fine gangue particles. The results in
Table 9 also indicate that concentrate regrinding resulted in reduced S content reporting to the CaF
2 concentrate. However, the S content was still above the market specification of 0.03%. Overall, concentrate regrinding had a detrimental effect of concentrate dilution by calcite as well as reduced CaF
2 recovery. It was also observed that with longer regrinding time, the total sulphur content increased. It is therefore clear that the processing of ore B without regrind was still the best, though sulphur content was still off-spec.
3.7. Effect of Lignin Sulphonate (Pionerra F250) Dosage on Sulphur Reduction for Ore B
Further flotation testwork was conducted using lignin sulphonate (Pionerra F250) as a sulphide depressant with no regrind with a view to reduce the S content to the industry specification of 0.03%. The depressant dosages were varied from 170 to 210 g/t and the results are presented in
Table 11. It is clear from
Table 11 that an increase in lignin sulphonate dosage resulted in a decrease in the total sulphur in the CaF
2 concentrate from 0.07% to 0.02%. Therefore, the acid-grace concentrate specifications were met in terms of both CaF
2 and S grades, although this was accompanied by a 7% loss in CaF
2 recovery. It can be concluded that the stage addition sulphide depressants in the rougher and cleaning stages in the processing of fluorite ores having high sulphur content is necessary to control the sulphur content in the concentrate.
The observed loss in CaF
2 recovery could be attributed to the depression of the sulphide minerals that are associated with the fluorite. To investigate this, mineralogical analysis was conducted on the concentrate to further understand the association of the minerals present in the ore, and the results are shown in
Table 9.
Table 9 shows that 37% of the sulphides were associated with fluorite and carbonates, while 7% of the sulphides were associated with other minerals. This observation shows that CaF
2 recovery loss was inevitable if the S content in the final concentrate is to be reduced using a sulphide depressant.