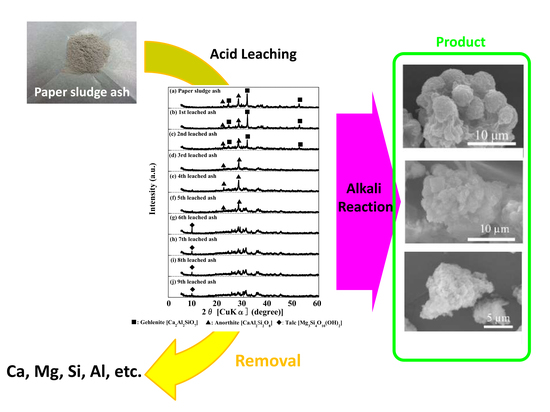
Effects of Step-Wise Acid Leaching with HCl on Synthesis of Zeolitic Materials from Paper Sludge Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Paper Sludge Ash
2.2. Step-Wise Acid Leaching
2.3. Zeolite Synthesis
2.4. Determination of Amount of Released Ca and Na, and Product CECs
3. Results and Discussion
3.1. Acid Leaching of Paper Sludge Ash
3.2. Zeolite Synthesis from Leached Ash and Procut Properties
4. Conclusions
Funding
Conflicts of Interest
References
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, N.; Ujike, I.; Kawaai, K.; Kawaguchi, T.; Yasuhara, H.; Nagae, T. Performance evaluation of low carbon concrete using paper sludge ash. J. MMIJ 2017, 133, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.; Lim, Y.T.; Kang, J.H.; So, S.; So, H. Influence of calcination and cooling conditions on pozzolanic reactivity of paper mill sludge. Constr. Build. Mater. 2018, 166, 257–270. [Google Scholar] [CrossRef]
- Segui, P.; Aubert, J.E.; Husson, B.; Measson, M. Characterization of wastepaper sludge ash for its valorization as a component of hydraulic binders. Appl. Clay Sci. 2012, 57, 79–85. [Google Scholar] [CrossRef]
- Mavroulidou, M.; Awoliyi, S. A study on the potential use of paper sludge ash in concrete with glass aggregate. Waste Manag. Res. 2018, 36, 1061–1065. [Google Scholar] [CrossRef] [Green Version]
- Bui, N.K.; Satomi, T.; Takahashi, H. Influence of industrial by-product and waste paper sludge ash on properties of recycles aggregate concrete. J. Clean. Prod. 2019, 214, 403–418. [Google Scholar] [CrossRef]
- Monower, S.; Hassan, A.-N.; William, A.; Linda, S.; Nicola, D. Analytical investigation of hydration mechanism of non-Portland binder with waste paper sludge ash. Constr. Build. Mater. 2019, 211, 80–87. [Google Scholar]
- Wajima, T.; Shimizu, T.; Ikegami, Y. Stabilization of mine waste using paper sludge ash under laboratory condition. Mater. Trans. 2007, 48, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Mavroulidou, M. Use of waste paper sludge ash as a calcium-based stabilizer for clay soils. Waste Manag. Res. 2018, 36, 1066–1072. [Google Scholar] [CrossRef]
- Wajima, T.; Rakovan, J.F. Removal of fluoride ions using calcined paper sludge. J. Therm. Anal. Calorim. 2013, 113, 1027–1035. [Google Scholar] [CrossRef]
- Wajima, T.; Rakovan, J.F. Removal behavior of phosphate from aqueous solution by calcined paper sludge. Colloid. Surf. A 2013, 435, 132–138. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umana, J.C.; Alastuey, A.; Hernandez, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Balfour, G.; Ndungu, P.; Gitari, W.M.; Hums, E. Synthesis of zeolites from coal fly ash: Application of a statistical experimental design. Res. Chem. Intermed. 2012, 38, 471–486. [Google Scholar] [CrossRef]
- Li, Y.; Liang, G.; Chang, L.; Zi, C.; Zhang, Y.; Peng, Z.; Zhao, W. Conversion of biomass ash to different types of zeolites: A review. Energy Sour. A 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Belviso, C. State-of-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Koshy, N.; Singh, D.N. Fly ash zeolites for water treatment applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, J.; Ma, H.; Ma, X.; Yuan, J. Synthesis of pure NaA zeolites from coal fly ashes for ammonium removal from aqueous solutions. Clean. Technol. Environ. Policy 2016, 18, 629–637. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, S.-W.; Kim, S.-S. Breakthrough analysis of carbon dioxide adsorption on zeolite synthesized from coal fly ash. Korean J. Chem. Eng. 2014, 31, 179–187. [Google Scholar] [CrossRef]
- Catalfamo, P.; Patane, G.; Primerano, P.; Pasquale, S.D.; Corigliano, F. The presence of calcium in the hydrothermal conversion of amorphous aluminosilicates into zeolite: Interference and removal. Mater. Eng. 1994, 5, 159–173. [Google Scholar]
- Murayama, N.; Ishimoto, H.; Shibata, J. Hydrothermal synthesis and physical property evaluation of zeolite from paper sludge ash. J. Min. Mater. Process. Inst. Jpn. 2000, 116, 31–36. [Google Scholar]
- Wajima, T.; Kuzawa, K.; Ishimoto, H.; Tamada, O.; Nishiyama, T. The synthesis of zeolite-P, Linde Type A, and hydroxysodalite zeolites from paper sludge ash at low temperature (80 °C): Optimal ash-leaching condition for zeolite synthesis. Am. Miner. 2004, 89, 1694–1700. [Google Scholar] [CrossRef]
- Wajima, T.; Ikegami, Y. Zeolite synthesis from paper sludge ash via acid leaching. Chem. Eng. Commun. 2008, 195, 305–315. [Google Scholar] [CrossRef]
- Wajima, T.; Munakata, K. Removal of Ca from paper sludge ash by acid leaching and synthesis of high cation exchange capacity zeolite material. Int. J. Soc. Mater. Eng. Resour. 2011, 18, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Wajima, T.; Munakata, K. Preparation of zeolitic material with simultaneous removal of NH4+ and PO43- from paper sludge ash via acid leaching. J. Mater. Cycles Waste Manag. 2014, 16, 367–372. [Google Scholar] [CrossRef]
- No. 111 Committee: Development of New Utilization of Materials (DNUM). In Natural Zeolite and Its Utilization; JSPS: Tokyo, Japan, 1994; pp. 318–325.







| Sample | Chemical Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | TiO2 | Na2O | K2O | |
| Paper sludge ash | 28.2 | 19.0 | 42.2 | 4.0 | 1.5 | 2.6 | 0.2 | 0.2 |
| 1st-leached ash | 34.5 | 23.0 | 31.6 | 3.9 | 1.8 | 3.1 | N.D. | N.D. |
| 2nd-leached ash | 37.5 | 24.0 | 25.1 | 3.8 | 2.2 | 3.8 | N.D. | N.D. |
| 3rd-leached ash | 45.7 | 21.7 | 18.8 | 3.7 | 2.6 | 4.8 | N.D. | N.D. |
| 4th-leached ash | 52.5 | 20.6 | 12.0 | 4.1 | 2.6 | 5.1 | N.D. | N.D. |
| 5th-leached ash | 62.6 | 14.0 | 8.5 | 4.6 | 2.2 | 5.5 | N.D. | N.D. |
| 6th-leached ash | 68.5 | 11.3 | 6.6 | 4.5 | 1.8 | 5.5 | N.D. | N.D. |
| 7th-leached ash | 70.4 | 10.5 | 5.7 | 4.6 | 1.7 | 5.3 | N.D. | N.D. |
| 8th-leached ash | 72.3 | 9.6 | 5.4 | 4.2 | 1.6 | 5.2 | N.D. | N.D. |
| 9th-leached ash | 73.2 | 9.3 | 5.2 | 4.1 | 1.5 | 5.1 | N.D. | N.D. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajima, T. Effects of Step-Wise Acid Leaching with HCl on Synthesis of Zeolitic Materials from Paper Sludge Ash. Minerals 2020, 10, 402. https://doi.org/10.3390/min10050402
Wajima T. Effects of Step-Wise Acid Leaching with HCl on Synthesis of Zeolitic Materials from Paper Sludge Ash. Minerals. 2020; 10(5):402. https://doi.org/10.3390/min10050402
Chicago/Turabian StyleWajima, Takaaki. 2020. "Effects of Step-Wise Acid Leaching with HCl on Synthesis of Zeolitic Materials from Paper Sludge Ash" Minerals 10, no. 5: 402. https://doi.org/10.3390/min10050402
APA StyleWajima, T. (2020). Effects of Step-Wise Acid Leaching with HCl on Synthesis of Zeolitic Materials from Paper Sludge Ash. Minerals, 10(5), 402. https://doi.org/10.3390/min10050402