A Highly Selective Reagent Scheme for Scheelite Flotation: Polyaspartic Acid and Pb–BHA Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Micro-Flotation Experiments
2.3. Zeta Potential Measurements
2.4. Fourier Transform Infrared Spectroscopy (FTIR) Measurements
2.5. X-Ray Photoelectron Spectroscopy (XPS) Measurements
3. Results and Discussion
3.1. Pure Mineral Flotation Results
3.2. Selective Adsorption of PASP on the Scheelite and Calcite Surfaces
3.2.1. Solution Chemistry Analysis of PASP
3.2.2. Zeta Potential Measurements
3.2.3. XPS Analysis Results
3.2.4. Crystal Chemistry Calculations
3.3. Effect of PASP on the Adsorption of Pb–BHA on the Mineral Surfaces
3.3.1. Zeta Potential Analysis Results
3.3.2. FTIR Analysis Results
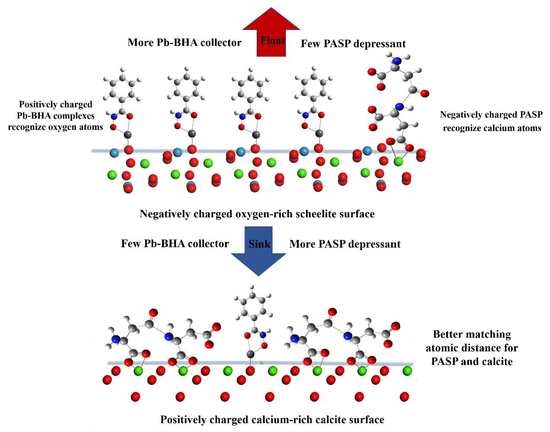
3.4. Adsorption Model of PASP and Pb–BHA on the Mineral Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rao, N.K. Beneficiation of tungsten ores in India: A review. Bull. Mater. Sci. 1996, 19, 201–265. [Google Scholar] [CrossRef] [Green Version]
- Ilhan, S.; Kalpakli, A.O.; Kahruman, C.; Yusufoglu, I. The investigation of dissolution behavior of gangue materials during the dissolution of scheelite concentrate in oxalic acid solution. Hydrometallurgy 2013, 136, 15–26. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Kelsall, G.H. The surface chemical properties of scheelite (CaWO4) I. The scheelite/water interface and CaWO4 solubility. Colloids Surf. 1987, 25, 369–385. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mechanism of fatty acid adsorption in salt-type mineral flotation. Miner. Eng. 1991, 4, 879–890. [Google Scholar]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; et al. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Han, H.; Xiao, Y.; Hu, Y.; Sun, W.; Nguyen, A.V.; Tang, H.; Gui, X.; Xing, Y.; Wei, Z.; Wang, J. Replacing Petrov’s process with atmospheric flotation using Pb-BHA complexes for separating scheelite from fluorite. Miner. Eng. 2020, 145, 1–10. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Z.; Han, H.; Sun, W.; Hu, Y.; Wang, J.; Wang, L.; Liu, H.; Yang, Y.; Zhang, C.; et al. Fluorite particles as a novel calcite recovery depressant in scheelite flotation using Pb-BHA complexes as collectors. Miner. Eng. 2019, 132, 84–91. [Google Scholar] [CrossRef]
- Han, H.; Liu, W.; Hu, Y.; Sun, W.; Li, X. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb-BHA complexes in Shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.L.; Sun, W.J.; Wang, J.J.; Gao, Z.Y.; Wang, L.; Zhang, C.Y.; et al. Selective Separation of Scheelite from Calcite by Self-Assembly of H2SiO3 Polymer Using Al3+ in Pb-BHA Flotation. Minerals 2019, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Bo, F.; Xianping, L.; Jinqing, W.; Pengcheng, W. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar] [CrossRef]
- Yang, Y.H.; Xu, L.H.; Tian, J.; Liu, Y.C.; Han, Y.X. Selective flotation of ilmenite from olivine using the acidified water glass as depressant. Int. J. Miner. Process. 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jiao, F.; Qin, W.Q.; Zhu, H.L.; Jia, W.H. Effect of acidified water glass on the flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Appl. Surf. Sci. 2018, 444, 747–756. [Google Scholar] [CrossRef]
- Deng, R.D.; Yang, X.F.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Ma, Y.Q. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Mercade, V. Effect of polyvalent metal-silicate hydrosols on the flotation of calcite. Trans. Soc. Min. Eng. AIME 1981, 268, 1842–1846. [Google Scholar]
- Yongxin, L.; Changgen, L. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. I. Selective flotation of scheelite. Int. J. Miner. Process. 1983, 10, 205–218. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.M.; Zhang, G.F.; Liu, D.Z.; Li, L.F. Selective flotation of scheelite from calcite using calcium lignosulphonate as depressant. Miner. Eng. 2018, 119, 73–75. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective flotation of scheelite from calcite using xanthan gum as depressant. Miner. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Jiao, F.; Dong, L.; Qin, W.; Liu, W.; Hu, C. Flotation separation of scheelite from calcite using pectin as depressant. Miner. Eng. 2019, 136, 120–128. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Yin, W.; Liang, X. Flotation separation of scheelite from calcite using carboxyl methyl cellulose as depressant. Miner. Eng. 2018, 127, 329–333. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiao, F.; Qin, W.; Zhu, H.; Jia, W. New insights into the carboxymethyl cellulose adsorption on scheelite and calcite: Adsorption mechanism, AFM imaging and adsorption model. Appl. Surf. Sci. 2019, 463, 105–114. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Han, H.; Sun, W.; Hu, Y. Improved flotation separation of cassiterite from calcite using a mixture of lead (II) ion/benzohydroxamic acid as collector and carboxymethyl cellulose as depressant. Miner. Eng. 2017, 113, 68–70. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Wu, Q.; Zheng, Y.; Cui, Y.; Yan, W.; Deng, J.; Peng, T. Comparative study on adsorption and depressant effects of carboxymethyl cellulose and sodium silicate in flotation. J. Mol. Liq. 2018, 268, 140–148. [Google Scholar] [CrossRef]
- Li, J.; Ping, Y.; Yunbai, L. Synthesis and scale inhibition of polyaspartic acid derivative. Ind. Water Treat. 2006, 26, 21–23. [Google Scholar]
- Liu, Z.; Sun, Y.; Zhou, X.; Wu, T.; Tian, Y.; Wang, Y. Synthesis and scale inhibitor performance of polyaspartic acid. J. Environ. Sci. 2011, 23, S153–S155. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Grant, C. Effect of Chelation Chemistry of Sodium Polyaspartate on the Dissolution of Calcite. Langmuir 2002, 18, 6813–6820. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Liu, Y. Flotation separation of smithsonite from calcite using 2-phosphonobutane-1,2,4-tricarboxylic acid as a depressant. Powder Technol. 2019, 352, 11–15. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.D.; Chen, K.F.; Zhu, Y.G.; Nguyen, A.V.; Tian, M.J.; Wang, L.; Yue, T.; et al. Novel catalysis catalysis mechanisms of benzohydroxamic acid adsorption by lead ions and changes in the-surface of scheelite particles. Miner. Eng. 2018, 119, 11–22. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W. Configurations of lead(II)–benzohydroxamic acid complexes in colloid and interface: A new perspective. J. Colloid Interface Sci. 2020, 562, 342–351. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Zhu, Y.; Li, B.; Song, Z. Structures of Pb-BHA Complexes Adsorbed on Scheelite Surface. Front. Chem. 2019, 7, 645. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef]
- Yue, T.; Han, H.; Hu, Y.; Wei, Z.; Wang, J.; Wang, L.; Sun, W.; Yang, Y.; Sun, L.; Liu, R.; et al. Beneficiation and Purification of Tungsten and Cassiterite Minerals Using Pb–BHA Complexes Flotation and Centrifugal Separation. Minerals 2018, 8, 566. [Google Scholar] [CrossRef] [Green Version]
- Silverman, D.; Kalota, D.; Stover, F. Effect of pH on corrosion inhibition of steel by polyaspartic acid. Corrosion 1995, 51, 818–825. [Google Scholar] [CrossRef]
- Kang, J.; Khoso, S.A.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Utilisation of 1-Hydroxyethylidene-1, 1-diphosphonicacid as a selective depressant for the separation of scheelite from calcite and fluorite. Colloid Surf. A 2019, 582. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Jiao, F.; Jia, W. Flotation separation of fluorite from calcite using polyaspartate as depressant. Miner. Eng. 2018, 120, 80–86. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Y.; Zhu, H.; Sun, W.; Qin, W.; Liu, R.; Gao, Z. Inhibition performance and adsorption of polycarboxylic acids in calcite flotation. Miner. Eng. 2019, 133, 60–68. [Google Scholar] [CrossRef]
- Capece, A.M.; Polk, J.E.; Shepherd, J.E. X-ray photoelectron spectroscopy study of BaWO4 and Ba2CaWO6. J. Electron. Spectrosc. Relat. Phenom. 2014, 197, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, G.-Y.; Li, C.-X.; Cheng, R.-J. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Li, C.W.; Sun, W.; Hu, Y.H. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Ai, G.; Liu, C.; Zhang, W. Utilization of sodium humate as selective depressants for calcite on the flotation of scheelite. Sep. Sci. Technol. 2018, 53, 2136–2143. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y.; Liu, X. Surface energies and appearances of commonly exposed surfaces of scheelite crystal. Trans. Nonferrous Met. Soc. China 2013, 23, 2147–2152. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, L.; Cui, X.; Hu, Y.; Sun, W.; Zeng, H. Probing Anisotropic Surface Properties and Surface Forces of Fluorite Crystals. Langmuir 2018, 34, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Hiçyilmaz, C.; Özbayoglu, G. The effects of amine and electrolytes on the zeta-potential of scheelite from Uludag, Turkey. Miner. Eng. 1992, 5, 945–951. [Google Scholar] [CrossRef]















| Samples | Elements | Atomic Concentration (%) | |
|---|---|---|---|
| Without Reagents | With PASP | ||
| Scheelite | C1s | 21.09 | 22.86 |
| O1s | 52.83 | 51.74 | |
| W4f | 12.57 | 11.61 | |
| Ca2p | 13.52 | 12.76 | |
| N1s | - | 1.04 | |
| Calcite | C1s | 31.18 | 27.14 |
| O1s | 52.36 | 56.58 | |
| Ca2p | 16.46 | 14.68 | |
| N1s | - | 1.60 | |
| Mineral Surface | Surface Unit Cell Area, nm2 | Number of Ca Atoms on Each Unit Cell Area | Ca Density, mol/m2 |
|---|---|---|---|
| Scheelite {101} | 0.328 | 1 | 5.06 |
| Scheelite {112} | 0.504 | 2 | 6.58 |
| Calcite {104} | 0.404 | 2 | 8.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Fu, J.; Han, H.; Sun, W.; Yue, T.; Wang, L.; Sun, L. A Highly Selective Reagent Scheme for Scheelite Flotation: Polyaspartic Acid and Pb–BHA Complexes. Minerals 2020, 10, 561. https://doi.org/10.3390/min10060561
Wei Z, Fu J, Han H, Sun W, Yue T, Wang L, Sun L. A Highly Selective Reagent Scheme for Scheelite Flotation: Polyaspartic Acid and Pb–BHA Complexes. Minerals. 2020; 10(6):561. https://doi.org/10.3390/min10060561
Chicago/Turabian StyleWei, Zhao, Junhao Fu, Haisheng Han, Wei Sun, Tong Yue, Li Wang, and Lei Sun. 2020. "A Highly Selective Reagent Scheme for Scheelite Flotation: Polyaspartic Acid and Pb–BHA Complexes" Minerals 10, no. 6: 561. https://doi.org/10.3390/min10060561
APA StyleWei, Z., Fu, J., Han, H., Sun, W., Yue, T., Wang, L., & Sun, L. (2020). A Highly Selective Reagent Scheme for Scheelite Flotation: Polyaspartic Acid and Pb–BHA Complexes. Minerals, 10(6), 561. https://doi.org/10.3390/min10060561