Effective Utilization of Limonitic Nickel Laterite via Pressurized Densification Process and Its Relevant Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physicochemical Properties of Raw Materials
2.2. Experimental Procedure
3. Results and Discussion
3.1. Influence of External Pressure Field on Limonitic Nickel Laterite Sintering
3.1.1. Effect of External Mechanical Pressure on Sintering Performance
3.1.2. Comparison of Metallurgical Performance of the Product Sinter
3.2. Intensification Mechanism of Pressurized Densification Sintering
3.2.1. Thermodynamic and Kinetic Conditions during Sintering
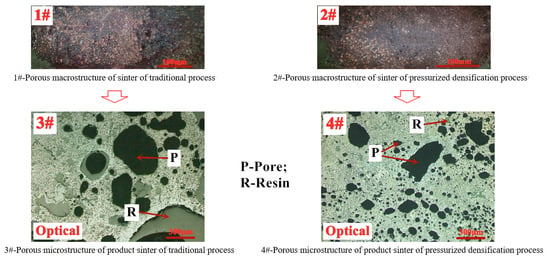
3.2.2. Sinter Porous Characters
3.2.3. Consolidation Characteristics
3.3. Industrial Application Prospect
4. Conclusions
- With the optimization of an external mechanical pressure on top of the sinter bed during limonitic nickel laterite sintering, the tumble index, and productivity are increased by 19.2% and 18.6%, respectively, and solid fuel rate is lowered by 10.3%. A great improvement in the sintering performance of limonitic laterite is achieved via pressurized densification sintering. Besides, the metallurgical performance remains excellent with RI and RDI+3.15mm of over 75% and 95%, respectively.
- The exhaust-gas temperature peak value is increased significantly with external mechanical pressure. In the meantime, excessive vertical sintering speed is under control due to the the densification of loose sinter. The application of an external pressure field promotes the synchronization of the heat front velocity and combustion front velocity during sintering and then better heat and mass transfer conditions.
- The mineralogy of the product sinter indicates that the external pressure field contributes to homogenizing and densifying the sinter microstructure during sintering with sinter porosity reduced by 42.4% and SFCA amount increased from 8.78% to 19.62%. The more efficient diffusion of particles in solid-phase and liquid-phase reactions leads to hercynite grains aggregation and growth and the formation of tighter interlocking texture between hercynite and SFCA. Pressurized densification sintering dramatically improves the microstructure and mineral compositions of product sinter and eventually achieves superior sintering performance of limonitic laterite.
- Industrial application of pressurized densification sintering process of limonitic laterite will be carried out in the sinter plant of one major Chinese stainless-steel enterprise. The sintering performance of limonitic laterite is expected to be improved significantly with the tumble index and the productivity both increased by 15% and solid fuel rate lowered by 10%. A total new economic benefit of 282.78 million RMB/a can be created. Thus, the pressurized densification sintering process should be identified as an effective technology not only obtaining better sintering performance of limonitic laterite but also bringing about remarkable economic benefits.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reck, B.K.; Rotter, V.S. Comparing growth rates of nickel and stainless steel use in the early 2000s. J. Ind. Ecol. 2012, 16, 518–528. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Pantelaki, O.; Kritikaki, A. Column leaching of Greek low-grade limonitic laterites. Minerals 2018, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Roki, F. Climate change policy to foster pollution prevention and sustainable industrial practices-A case study of the global nickel industry. Miner. Eng. 2012, 39, 196–205. [Google Scholar]
- Thorne, R.; Roberts, S.; Herrington, R. Climate change and the formation of nickel laterite deposits. Geology 2012, 40, 331–334. [Google Scholar] [CrossRef]
- Orberger, B.; Van Der Ent, A. Nickel laterites as sources of nickel, cobalt and scandium: Increasing resource efficiency through new geochemical and biological insights. J. Geochem. Explor. 2019, 204, 297–299. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Zhang, F. Mineralogical characteristics and preliminary beneficiation of nickel slag from reduction roasting-ammonia leaching. Minerals 2017, 7, 98. [Google Scholar]
- Cracknell, M.J.; Jansen, N.H. National virtual core library hylogging data and ni–co laterites: A mineralogical model for resource exploration, extraction and remediation. Aust. J. Earth Sci. 2016, 63, 1053–1067. [Google Scholar] [CrossRef]
- Isatelle, F.; Rivoirard, J. Mineral resources classification of a nickel laterite deposit: Comparison between conditional simulations and specific areas. J. S. Afr. Inst. Min. Met. 2019, 119, 871–882. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pickles, C.A.; Peacey, J.; Elliott, R.; Forster, J. Factors affecting the upgrading of a nickeliferous limonitic laterite ore by reduction roasting, thermal growth and magnetic separation. Minerals 2017, 7, 176. [Google Scholar] [CrossRef] [Green Version]
- Mudd, G.M.; Jowitt, S.M. A detailed assessment of global nickel resource trends and endowments. Econ. Geology 2014, 109, 1813–1841. [Google Scholar] [CrossRef]
- Eckelman, M.J. Facility-level energy and greenhouse gas life-cycle assessment of the global nickel industry. Resour. Conserv. Recycl. 2010, 54, 256–266. [Google Scholar] [CrossRef]
- Moskalyk, R.; Alfantazi, A. Nickel laterite processing and electrowinning practice. Miner. Eng. 2002, 15, 593–605. [Google Scholar] [CrossRef]
- Liu, P.; Li, B.; Cheung, S.C.; Wu, W. Material and energy flows in rotary kiln-electric furnace smelting of ferronickel alloy with energy saving. Appl. Therm. Eng. 2016, 109, 542–559. [Google Scholar] [CrossRef]
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- Tsuji, H. Behavior of reduction and growth of metal in smelting of saprolite Ni-ore in a rotary kiln for production of ferro-nickel alloy. ISIJ Int. 2012, 52, 1000–1009. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Pan, J.; Zhu, D.; Yang, C.; Guo, Z.; Xue, Y. Improved beneficiation of nickel and iron from a low-grade saprolite laterite by addition of limonitic laterite ore and CaCO3. J. Mater. Res. Technol. 2020, 9, 2578–2589. [Google Scholar] [CrossRef]
- Lv, X.W.; Bai, C.G.; He, S.P.; Huang, Q.Y. Mineral change of Philippine and Indonesia nickel lateritic ore during sintering and mineralogy of their sinter. ISIJ Int. 2010, 50, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Xue, Y.; Pan, J.; Yang, C.; Guo, Z.; Tian, H.; Liao, H.; Pan, L.; Huang, X. An investigation into the distinctive sintering performance and consolidation mechanism of limonitic laterite ore. Powder Technol. 2020, 367, 616–631. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, H.B.; Zhang, J.L.; Xu, C.F.; Du, S. Analysis of application of high proportion laterite nickel ore in sinter production. Sinter. Pellet. 2013, 38, 6–9. (In Chinese) [Google Scholar]
- Panychev, A.A. Potential for the use of low-grade limonite ores from dumps. Metallurgist 2006, 50, 45–48. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Usui, T. Summarized achievements of the porous meso-mosaic texture sinter research project. ISIJ Int. 2005, 45, 414–426. [Google Scholar] [CrossRef]
- Landers, M.; Gilkes, R. Dehydroxylation and dissolution of nickeliferous goethite in New Caledonian lateritic Ni ore. Appl. Clay Sci. 2007, 35, 162–172. [Google Scholar] [CrossRef]
- Landers, M.; Gilkes, R.; Wells, M. Dissolution kinetics of dehydroxylated nickeliferous goethite from limonitic lateritic nickel ore. Appl. Clay Sci. 2009, 42, 615–624. [Google Scholar] [CrossRef]
- Li, T.; Sun, C.; Liu, X.; Song, S.; Wang, Q. The effects of MgO and Al2O3 behaviours on softening? Melting properties of high basicity sinter. Ironmak. Steelmak. 2018, 45, 755–763. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Hong, Z.; Zhang, W.; Zhou, H.; Kou, M. The mechanism of the effect of Al2O3 content on the liquid phase fluidity of iron ore fines. Processes 2019, 7, 931. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, T.; Deodar, A.V.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of alumina on iron ore sinter properties and productivity in the conventional and selective granulation sintering process. Steel Res. Int. 2009, 80, 686–692. [Google Scholar]
- Pownceby, M.I.; Webster, N.A.S.; Manuel, J.R.; Ware, N. The influence of ore composition on sinter phase mineralogy and strength. Miner. Process. Extr. Met. 2016, 125, 140–148. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Matsumura, M. Development of sinter quality and the technology with corresponding to the change of iron ore resources: 100 years of sintering process and to the future. Etsu Hagane J. Iron Steel Inst. Jpn. 2014, 100, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, T.; Sah, R.; Mahapatra, P.C. Influence of sinter basicity (CaO/SiO2) on low and high alumina iron ore sinter quality. Miner. Process. Extr. Met. 2014, 123, 75–85. [Google Scholar] [CrossRef]
- De Magalhaes, M.S.; Brandao, P.R.G. Microstructures of industrial sinters from Quadrilatero Ferrifero’s iron ores, Minas Gerais State Brazil. Miner. Eng. 2003, 16, 1251–1256. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, D.Q.; Pan, J. Sintering Performance of Blends Containing High Proportion of Limonite. In Proceedings of the 8th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 26 February–2 March 2017. [Google Scholar]
- Liu, D.-H.; Zhang, J.; Xue, X.; Wang, G.; Kang, Q.-F. Basic characteristics of Australian iron ore concentrate and its effects on sinter properties during the high-limonite sintering process. Int. J. Miner. Met. Mater. 2017, 24, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Wang, Z.H.; Wang, J.C.; Zhou, F.Q.; Quan, Q. Experiment research on limonitic laterite ore sintering. Ferro. Alloys 2015, 11, 21–24. (In Chinese) [Google Scholar]
- Pan, L.T. Laterite nickel ore sintering production practice research. Ferro. Alloys 2013, 2, 7–10. (In Chinese) [Google Scholar]
- Loo, C.E.; Heikkinen, J. Structural transformation of beds during iron ore sintering. ISIJ Int. 2012, 52, 2158–2167. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.Q.; Xue, Y.X.; Pan, J.; Yang, C.C.; Guo, Z.Q.; Tian, H.Y.; Wang, D.Z. Strengthening Sintering of Limonitic Nickel Laterite by Substituting Ferronickel Tailings for Sintering Fluxes. In Proceedings of the 11th International Symposium on High-Temperature Metallurgical Processing, San Diego, CA, USA, 23–27 February 2020. [Google Scholar]
- Umadevi, T.; Deodhar, A.; Mahapatra, P.; Prabhu, M.; Ranjan, M. Influence of coating granulation process on iron ore sinter quality and productivity. Steel Res. Int. 2010, 81, 716–723. [Google Scholar] [CrossRef]
- Zhu, D.; Shi, B.; Pan, J.; Zhang, F. Effect of pre-briquetting on the granulation of sinter mixture containing high proportion of specularite concentrate. Powder Technol. 2018, 331, 250–257. [Google Scholar] [CrossRef]
- Contreras, J.; Castillo, G.; Rodríguez, E.A.; Das, T.; Guzmán, A. Microstructure and properties of hercynite–magnesia–calcium zirconate refractory mixtures. Mater. Charact. 2005, 54, 354–359. [Google Scholar] [CrossRef]
- Zhao, J.; Loo, C.E. Dependence of flame front speed on iron ore sintering conditions. Miner. Process. Extr. Met. 2016, 125, 165–171. [Google Scholar] [CrossRef]
- Ono, H.; Dohi, Y.; Arikata, Y.; Usui, T. Effect of mineral composition and pore structure on reducibility of composite iron ore sinter. ISIJ Int. 2009, 49, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Chou, J.; Shi, B.; Pan, J. Influence of MgO on low temperature reduction and mineralogical changes of sinter in simulated COREX shaft furnace reducing conditions. Minerals 2019, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Z.; Liu, Z.; Zhang, J. Characteristics of combustion zone and evolution of mineral phases along bed height in ore sintering. Int. J. Miner. Met. Mater. 2017, 24, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Loo, C.E.; Tame, N.; Penny, G.C. Effect of iron ores and sintering conditions on flame front properties. ISIJ Int. 2012, 52, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, J.; Hosotani, Y. Effect of mineralogical properties of iron ore on pore formation of sinter. Tetsu Hagane 2001, 87, 298–304. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, B.Y.; Li, Z.J.; Qu, D.L.; Xu, N.; Li, L.S. Hot-pressing sintering performance of block magnesite ore. J. Chin. Ceram. Soc. 2014, 42, 1600–1604. (In Chinese) [Google Scholar]
- Liu, D.; Evans, G.; Loo, C.E. Iron ore sinter structure development under realistic thermal conditions. Chem. Eng. Res. Des. 2018, 130, 129–137. [Google Scholar] [CrossRef]
- Liu, D.; Loo, C.E.; Evans, G. Flow characteristics of the molten mix generated during iron ore sintering. Int. J. Miner. Process. 2016, 149, 56–68. [Google Scholar] [CrossRef]
- Sokol, E.; Sharygin, V.; Kalugin, V.; Volkova, N.; Nigmatulina, E. Fayalite and kirschsteinite solid solutions in melts from burned spoil-heaps, South Urals, Russia. Eur. J. Miner. 2002, 14, 795–807. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Churchill, J.G.; Tufaile, F.; Pownceby, M.I.; Manuel, J.R.; Kimpton, J.A. Fundamentals of Silico-Ferrite of Calcium and Aluminium (SFCA) and SFCA-I Iron Ore Sinter Bonding Phase Formation: Effects of Titanomagnetite-based Ironsand and Titanium Addition. ISIJ Int. 2016, 56, 1715–1722. [Google Scholar] [CrossRef] [Green Version]












| Area No. | Elemental Compositions (Atomic Conc, %) | Mineral Phases | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cr | Ni | Mg | Al | Si | Ca | O | ||
| 1 | 31.59 | 0.59 | 0.26 | 0.45 | 2.72 | 1.21 | 0.78 | 62.40 | Goethite |
| 2 | 35.27 | 0.67 | 0.34 | 0.89 | 4.28 | 0.68 | 0.29 | 57.58 | Goethite |
| 3 | 40.36 | 0.62 | 0.13 | 0.22 | 0.85 | 0.21 | 0.18 | 57.43 | Hematite |
| 4 | 38.91 | 0.23 | 0.15 | 0.69 | 0.79 | 0.11 | 0.21 | 58.91 | Maghemite |
| 5 | 20.40 | 16.34 | - | 2.12 | 2.49 | 0.33 | 0.27 | 58.05 | Chromite spinel |
| 6 | 0.11 | - | - | 0.08 | 0.12 | 32.67 | 0.14 | 66.88 | Stishovite |
| 7 | 0.98 | - | - | 21.37 | 0.03 | 18.96 | 0.28 | 58.38 | Enstatite |
| Pressure (Pa) | Tumble Index (%) | Productivity (t·m−2·h−1) | Solid Fuel Rate (kg/t) |
|---|---|---|---|
| 0.00 | 45.87 | 0.97 | 140.52 |
| 1561 | 50.40 | 1.09 | 130.24 |
| 3121 | 50.93 | 1.11 | 128.81 |
| 4682 | 54.27 | 1.13 | 127.26 |
| 6242 | 54.67 | 1.15 | 126.12 |
| 7803 | 53.87 | 1.08 | 128.97 |
| Sintering Processes | Fetotal | FeO | NiO | Cr2O3 | SiO2 | CaO | Al2O3 | MgO |
|---|---|---|---|---|---|---|---|---|
| Traditional sintering process [18] | 43.95 | 21.15 | 1.08 | 3.36 | 7.69 | 10.79 | 4.89 | 6.65 |
| Pressurized densification sintering process | 43.73 | 20.59 | 1.09 | 3.68 | 7.76 | 10.83 | 4.85 | 6.61 |
| Area No. | Elemental Compositions (Atomic Conc, %) | Mineral Phases | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cr | Ni | Mg | Al | Si | Ca | O | ||
| 1 | 34.02 | 0.23 | 0.16 | 4.78 | 0.56 | 0.43 | 0.32 | 59.50 | Hercynite |
| 2 | 35.27 | 0.14 | 0.09 | 3.69 | 5.21 | 0.54 | 0.27 | 54.79 | Hercynite |
| 3 | 16.78 | 17.25 | - | 3.72 | 6.33 | 0.22 | 0.13 | 55.57 | Chromite spinel |
| 4 | 12.27 | 0.08 | 0.11 | 4.56 | 0.36 | 11.24 | 12.79 | 58.59 | Eutectic olivine phase |
| 5 | 13.89 | 0.05 | 0.07 | 0.23 | 6.69 | 12.31 | 14.57 | 52.19 | Eutectic olivine phase |
| 6 | 34.65 | 0.46 | 0.29 | 0.66 | 6.77 | 0.35 | 0.21 | 56.61 | Hercynite |
| 7 | 11.39 | 0.06 | 0.08 | 4.35 | 5.27 | 10.98 | 13.36 | 54.51 | Eutectic olivine phase |
| 8 | 31.74 | 0.05 | 16.59 | 0.35 | 0.52 | 0.12 | 0.09 | 50.54 | Nickel-ferric spinel |
| 9 | 33.68 | 0.13 | 0.25 | 5.39 | 0.45 | 0.15 | 0.12 | 59.83 | Hercynite |
| 10 | 30.37 | 0.31 | 0.18 | 0.68 | 4.54 | 4.33 | 7.68 | 51.91 | SFCA |
| 11 | 26.56 | 0.19 | 0.13 | 0.88 | 6.82 | 5.36 | 8.22 | 51.84 | SFCA |
| 12 | 31.26 | 0.17 | 0.09 | 0.51 | 3.79 | 5.33 | 7.69 | 51.16 | SFCA |
| Area No. | Elemental Compositions (Atomic Conc, %) | Mineral Phases | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cr | Ni | Mg | Al | Si | Ca | O | ||
| 1 | 12.42 | 21.53 | 0.11 | 3.98 | 6.78 | 0.14 | 0.33 | 54.71 | Chromite spinel |
| 2 | 32.95 | 0.72 | 0.44 | 3.78 | 7.07 | 0.13 | 0.33 | 54.58 | Hercynite |
| 3 | 37.61 | 0.17 | 0.51 | 4.92 | 0.52 | 0.18 | 0.51 | 55.58 | Hercynite |
| 4 | 10.34 | 0.11 | 0.05 | 2.12 | 6.15 | 13.21 | 15.80 | 52.22 | Eutectic olivine phase |
| 5 | 34.98 | 0.23 | 0.35 | 0.69 | 6.75 | 0.19 | 0.48 | 56.33 | Hercynite |
| 6 | 34.39 | 0.52 | 0.10 | 5.30 | 0.24 | 0.19 | 0.33 | 58.93 | Hercynite |
| 7 | 35.28 | 0.28 | 17.54 | 0.80 | 0.31 | 0.20 | 0.27 | 45.32 | Nickel-ferric spinel |
| 8 | 37.99 | 0.84 | 0.33 | 4.92 | 2.55 | 0.12 | 0.59 | 52.66 | Hercynite |
| 9 | 28.53 | 0.72 | 0.12 | 0.81 | 5.61 | 4.52 | 6.99 | 52.70 | SFCA |
| 10 | 34.32 | 0.45 | 0.14 | 0.68 | 3.69 | 4.07 | 6.45 | 50.20 | SFCA |
| 11 | 35.21 | 0.63 | 0.11 | 0.85 | 3.36 | 4.29 | 6.77 | 48.78 | SFCA |
| No. | Solid Phases | Liquid Phases | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hercynite | Chromite Spinel | Nickel-Ferric Spinel | Eutectic Olivine Phases | SFCA | |||||
| H | H-1 | H-2 | K | K-1 | K-2 | ||||
| A [18] | 17.14 | 20.81 | 23.24 | 2.55 | 1.05 | 17.23 | 2.13 | 7.07 | 8.78 |
| B | 23.28 | 15.78 | 16.36 | 3.09 | 1.18 | 20.69 | - | - | 19.62 |
| Sinter Indices | Tumble Index (%) | Productivity (t·m−2·h−1) | Solid Fuel (kg/t) |
|---|---|---|---|
| Traditional sintering process [18] | 51.07 | 0.90 | 161.04 |
| Pressurized densification sintering process | 58.73 | 1.04 | 144.94 |
| Technical Benefits | Economic Benefits | |||||||
|---|---|---|---|---|---|---|---|---|
| Proportions (%) | Variations (Thousand t/a) | Unit Prices (RMB/t) | New Profits (Million RMB/a) | |||||
| Productivity Improvement | Solid Fuel Rate Reduction | Production Increase | Anthracite Consumption Reduction | Product Sinter | Anthracite | Sinter Production | Anthracite Consumption | Total |
| 15 | 10 | 300 | 37.04 | 850 | 750 | 255 | 27.78 | 282.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhu, D.; Pan, J.; Guo, Z.; Yang, C.; Tian, H.; Duan, X.; Huang, Q.; Pan, L.; Huang, X. Effective Utilization of Limonitic Nickel Laterite via Pressurized Densification Process and Its Relevant Mechanism. Minerals 2020, 10, 750. https://doi.org/10.3390/min10090750
Xue Y, Zhu D, Pan J, Guo Z, Yang C, Tian H, Duan X, Huang Q, Pan L, Huang X. Effective Utilization of Limonitic Nickel Laterite via Pressurized Densification Process and Its Relevant Mechanism. Minerals. 2020; 10(9):750. https://doi.org/10.3390/min10090750
Chicago/Turabian StyleXue, Yuxiao, Deqing Zhu, Jian Pan, Zhengqi Guo, Congcong Yang, Hongyu Tian, Xi Duan, Qingzhou Huang, Liaoting Pan, and Xuezhong Huang. 2020. "Effective Utilization of Limonitic Nickel Laterite via Pressurized Densification Process and Its Relevant Mechanism" Minerals 10, no. 9: 750. https://doi.org/10.3390/min10090750
APA StyleXue, Y., Zhu, D., Pan, J., Guo, Z., Yang, C., Tian, H., Duan, X., Huang, Q., Pan, L., & Huang, X. (2020). Effective Utilization of Limonitic Nickel Laterite via Pressurized Densification Process and Its Relevant Mechanism. Minerals, 10(9), 750. https://doi.org/10.3390/min10090750