Application of the Mineralogy and Mineral Chemistry of Carbonates as a Genetic Tool in the Hydrothermal Environment
Abstract
:1. Introduction
2. Samples and Analytical Techniques
3. Geological Setting and Mineralogical Features
3.1. Palai-Islica, Southeastern Spain
3.2. El Dorado, La Serena, Central Chile
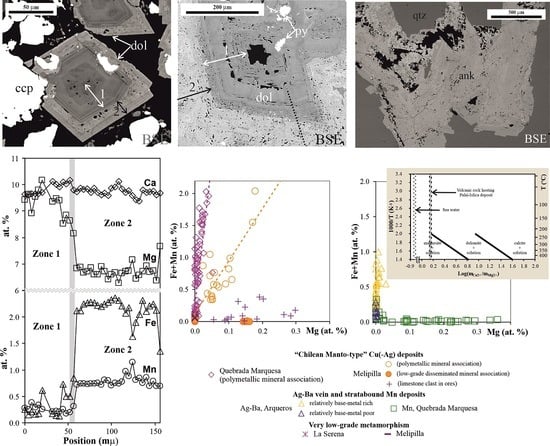
3.3. Quebrada Marquesa and Arqueros, La Serena, Central Chile
3.4. Melipilla, Central Chile
4. Elemental Composition of Carbonate Minerals
4.1. Epithermal Au-Cu Volcanic-Hosted Deposits, Palai-Islica and El Dorado
4.2. “Chilean Manto-Type” Cu(-Ag) Deposits, Quebrada Marquesa in La Serena and Melipilla
4.3. Other Deposits: Ag-Ba Vein and Stratabound Mn Deposits (Quebrada Marquesa and Arqueros in La Serena)
4.4. Very Low-Grade Metamorphism (La Serena and Melipilla)
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polgari, M.; Okita, P.M.; Hein, J.R. Stable isotope evidence for the origin of the Úrkút manganese ore deposit, Hungary. J. Sediment. Res. 1991, 61, 384–393. [Google Scholar] [CrossRef]
- Spangenberg, J.; Fontbote, L.; Sharp, Z.D.; Hunziker, J. Carbon and oxygen isotope study of hydrothermal carbonates in the zinc-lead deposits of the San Vicente district, central Peru: A quantitative modeling on mixing processes and CO2 degassing. Chem. Geol. 1996, 133, 289–315. [Google Scholar] [CrossRef]
- Bouzenoune, A.; Lecolle, P. Petrographic and geochemical arguments for hydrothermal formation of the Ouenza siderite deposit (NE Algeria). Mineral. Depos. 1997, 32, 189–196. [Google Scholar] [CrossRef]
- Gianelli, G.; Ruggieri, G.; Mussi, M. Isotopic and fluid-inclusion study of hydrothermal and metamorphic carbonates in the Larderello geothermal field and surrounding areas, Italy. Geothermics 1997, 26, 393–417. [Google Scholar] [CrossRef]
- Castorina, F.; Masi, U. Sr-isotopic composition of siderite for assessing the origin of mineralizing fluid: The case study from the Jebel Awam deposit (Central Morocco). Ore Geol. Rev. 2000, 17, 83–89. [Google Scholar] [CrossRef]
- Wilkinson, J.J.; Earls, G. A high-temperature hydrothermal origin for black dolomite matrix breccias in the Irish Zn-Pb orefield. Mineral. Mag. 2000, 64, 1017–1036. [Google Scholar] [CrossRef]
- Lavric, J.; Spangenberg, J. Stable isotope (C, O, S) systematics of the mercury mineralization at Idrija, Slovenia: Constraints on fluid source and alteration processes. Mineral. Depos. 2003, 38, 886–899. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.J.; Everett, C.E.; Boyce, A.J.; Gleeson, S.A.; Rye, D.M. Intracratonic crustal seawater circulation and the genesis of subseafloor zinc-lead mineralization in the Irish orefield. Geology 2005, 33, 805–808. [Google Scholar] [CrossRef]
- Schroll, E.; Koppel, V.; Cerny, I. Pb and Sr isotope and geochemical data from the Pb-Zn deposit Bleiberg (Austria): Constraints on the age of mineralization. Mineral. Petrol. 2006, 86, 129–156. [Google Scholar] [CrossRef]
- Song, Y.; Hou, Z.; Cheng, Y.; Yang, T.; Xue, C. Fluid inclusion and isotopic constraints on the origin of ore-forming fluid of the Jinman–Liancheng vein Cu deposit in the Lanping Basin, western Yunnan, China. Geofluids 2016, 16, 56–77. [Google Scholar] [CrossRef] [Green Version]
- Marques de Sá, C.; Noronha, F.; Cardellach, E.; Bobos, I. Fluid inclusion and (S, C, O, Pb) isotope study of Pb-Zn-(Cu-Ag) hydrothermal veins from Central and Northern Portugal–Metallogenic implications. Ore Geol. Rev. 2019, 112, 1–15. [Google Scholar] [CrossRef]
- Moroni, M.; Rossetti, P.; Naitza, S.; Magnani, L.; Ruggieri, G.; Aquino, A.; Tartarotti, P.; Franklin, A.; Ferrari, E.; Castelli, D.; et al. Factors controlling hydrothermal nickel and cobalt mineralization—Some suggestions from historical ore deposits in Italy. Minerals 2019, 9, 429. [Google Scholar] [CrossRef] [Green Version]
- Maghfouri, S.; Hosseinzadeh, M.R.; Lentz, D.R.; Tajeddin, H.A.; Movahednia, M.; Shariefi, A. Nature of ore-forming fluids in the Mehdiabad world-class sub-seafloor replacement SEDEX-type Zn-Pb-Ba-(Cu-Ag) deposit, Iran; constraints from geochemistry, fluid inclusions, and O-C-Sr isotopes. J. Asian Earth Sci. 2021, 207, 104654. [Google Scholar] [CrossRef]
- Frondel, C. The Minerals of Franklin and Sterling Hill, a Checklist; John Wiley & Sons: New York, NY, USA, 1972; 94p. [Google Scholar]
- Jebrak, M. Le district filonien a Pb-Zn-Ag et carbonates du Jebel Aouam (Maroc central). Bull. Mineral. 1985, 108, 487–498. [Google Scholar] [CrossRef]
- Polgari, M.; Forizs, I. Distribution of Mn in carbonates from the Upony Mts., NE-Hungary. Geol. Carpathica 1996, 47, 215–225. [Google Scholar]
- Akçay, M.; Özkan, H.M.; Spiro, B.; Wilson, R.; Hoskin, P.W.O. Geochemistry of a high-T hydrothermal dolostone from the Emirli (Ödemis, western Turkey) Sb-Au deposit. Mineral. Mag. 2003, 67, 671–688. [Google Scholar] [CrossRef]
- Russell, M.J. Manganese halo surrounding the Tynagh ore deposit, Ireland: A preliminary note. Trans. Inst. Min. Metall. 1974, 83, B65–B66. [Google Scholar]
- Gwosdz, W.; Krebs, W. Manganese halo surrounding Meggan ore deposit, Germany. Trans. Inst. Min. Metall. 1977, 86, B73–B77. [Google Scholar]
- Cruset, D.; Cantarero, I.; Vergés, J.; John, C.M.; Muñoz-López, D.; Travé, A. Changes in fluid regime in syn-orogenic sediments during the growth of the south Pyrenean fold and thrust belt. Glob. Planet. Chang. 2018, 171, 207–224. [Google Scholar] [CrossRef]
- Motte, G.; Hoareau, G.; Callot, J.-P.; Révillon, S.; Piccoli, F.; Calassou, S.; Gaucher, E.C. Rift and salt-related multi-phase dolomitization: Example from the northwestern Pyrenees. Mar. Pet. Geol. 2021, 126, 104932. [Google Scholar] [CrossRef]
- Cruset, D.; Ibáñez-Insa, J.; Cantarero, I.; John, C.M.; Travé, A. Significance of fracture-filling rose-like calcite crystal clusters in the SE pyrenees. Minerals 2020, 10, 522. [Google Scholar] [CrossRef]
- Morales Ruano, S.; Carrillo Rosúa, F.J.; Fenoll Hach-Alí, P.; de la Fuente Chacón, F.; Contreras López, E. Epithermal Cu-Au mineralization in the Palai-Islica deposit, Almería, southeastern Spain: Fluid inclusion evidence of mixing of fluids as guide to gold mineralization. Can. Mineral. 2000, 38, 553–566. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Rosúa, F.J.; Morales-Ruano, S.; Fenoll Hach-Ali, P. Iron sulphides at the epithermal gold-copper deposit of Palai-Islica (Almeriía, SE Spain). Mineral. Mag. 2003, 67, 1059–1080. [Google Scholar] [CrossRef]
- Carrillo-Rosúa, F.J.; Morales Ruano, S.; Morata Céspedes, D. Mineral features of Cu-Ag-Ba-Mn mineralisations of La Serena, Chile. In Mineral Exploration and Sustainable Development; Eliopoulos, D.G., Eilu, P., Economou-Eliopoulos, M., Damigos, D., Christidis, G., Brown, A., Bouchot, V., Borg, G., Boni, M., Benardos, A., et al., Eds.; Millpress: Rotterdam, The Netherlands, 2003; Volume 2, pp. 953–956. [Google Scholar]
- Carrillo-Rosúa, J.; Morales-Ruano, S.; Fenoll Hach-Alí, P. Textural and chemical features of sphalerite from the Palai-Islica deposit (SE Spain): Implications for ore genesis and color. Neues Jahrb. Mineral. Abh. 2008, 185, 63–78. [Google Scholar] [CrossRef]
- Carrillo-Rosúa, J.; Morales-Ruano, S.; Esteban-Arispe, I.; Fenoll Hach-Alí, P. Significance of phyllosilicate mineralogy and mineral chemistry in an epithermal environment. Insights from the Palai-Islica Au-Cu deposit (Almería, SE Spain). Clays Clay Miner. 2009, 57, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Rosúa, J. El Depósito Epitermal de Oro-Cobre Palai-Islica (Carboneras, Almería). Mineralogía, Geoquímica y Metalogenia. Unpublished Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2005; 421p. [Google Scholar]
- Carrillo-Rosúa, J.; Morales-Ruano, S.; Morata, D.; Boyce, A.J.; Belmar, M.; Fallick, A.E.; Fenoll Hach-Alí, P. Mineralogy and geochemistry of El Dorado epithermal gold deposit, El Sauce district, central-northern Chile. Mineral. Petrol. 2008, 92, 341–360. [Google Scholar] [CrossRef]
- Boric, R. Geología y yacimientos metálicos del Distrito Talcuna, Región de Coquimbo. Andean Geol. 1985, 25-26, 57–75. [Google Scholar]
- Oyarzun, R.; Ortega, L.; Sierra, J.; Lunar, R.; Oyarzun, J. Cu, Mn and Ag mineralization in the Quebrada Marquesa Quadrangle: The Talcuna and Arqueros district. Mineral. Depos. 1998, 33, 547–559. [Google Scholar] [CrossRef]
- Carrillo-Rosúa, F.J.; Morales Ruano, S.; Morata, D.; Belmar, M.; Fenoll Hach-Alí, P. Fluidos relacionados con diferentes mineralizaciones de Cu-Ag y metamorfismo de bajo grado en el área de la Serena (Cordillera de la Costa, Chile Central). Datos preliminares. Macla 2004, 2, 89–90. [Google Scholar]
- Carrillo-Rosúa, J.; Boyce, A.J.; Morales-Ruano, S.; Morata, D.; Roberts, S.; Munizaga, F.; Moreno-Rodríguez, V. Extremely negative and inhomogeneous sulfur isotope signatures in Cretaceous Chilean manto-type Cu–(Ag) deposits, Coastal Range of central Chile. Ore Geol. Rev. 2014, 56, 13–24. [Google Scholar] [CrossRef]
- Turner, S.P.; Platt, J.P.; George, R.M.M.; Kelley, S.P.; Pearson, D.G.; Nowell, G.M. Magmatism associated with orogenic collapse of the Betic-Alboran domain, SE Spain. J. Petrol. 1999, 40, 1011–1036. [Google Scholar] [CrossRef]
- Carrillo-Rosúa, F.J.; Morales Ruano, S.; Fenoll Hach-Alí, P. The three generations of gold in the Palai-Islica epithermal deposit, southeastern Spain. Can. Mineral. 2002, 40, 1465–1481. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, L.; Egert, E. Cuadrángulo Quebrada Marquesa, provincia de Coquimbo. Carta Geol. de Chile Inst. de Investig. Geol. 1965, 15, 92. [Google Scholar]
- Morata, D.; Féraud, G.; Aguirre, L.; Arancibia, G.; Belmar, M.; Morales, S.; Carrillo, J. Geochronology of the lower cretaceous volcanism from the Coastal Range (29°20′–30°S), Chile. Rev. Geol. Chile 2008, 35, 123–145. [Google Scholar] [CrossRef]
- Morata, D.; Aguirre, L. Extensional Lower Cretaceous volcanism in the Costal Range (29°20′–30°00′ S) Chile: Geochemistry and petrogenesis. J. S. Am. Earth Sci. 2003, 16, 459–476. [Google Scholar] [CrossRef]
- Morata, D.; Aguirre, L.; Belmar, M.; Morales, S. Constraining very low-grade metamorphic conditions based on prehnite chemistry. In Proceedings of the Congreso Geológico Chileno, Concepción, Chile, 6–10 October 2003. [Google Scholar]
- Rutter, E.H.; Faulkner, D.R.; Burgess, R. Structure and geological history of the Carboneras Fault Zone, SE Spain: Part of a stretching transform fault system. J. Struct. Geol. 2012, 45, 68–71. [Google Scholar] [CrossRef]
- Abad, I.; Jiménez-Millán, J.; Schleicher, A.M.; van der Pluijm, B.A. Mineral characterization, clay quantification and Ar-Ar dating of faulted schists in the Carboneras and Palomares Faults (Betic Cordillera, SE Spain). Eur. J. Mineral. 2017, 29, 17–34. [Google Scholar] [CrossRef]
- Bellon, H.; Bordet, P.; Montenat, C. Chronologie du magmatisme Néogène des Cordillères Bètiques (Espagne mèridionale). Bull. Soc. Geol. France 1983, 25, 205–217. [Google Scholar] [CrossRef]
- Rosenberg, P.E.; Holland, H.D. Calcite-dolomite magnesite stability relations in solutions at elevated temperatures. Science 1964, 145, 700–701. [Google Scholar] [CrossRef]
- Garrels, R.M.; Thompson, M.E. A chemical model for sea water at 25 °C and one atm. total pressure. Am. J. Sci. 1962, 260, 57–66. [Google Scholar] [CrossRef]
- Fernández Soler, J.M. El Vulcanismo Calco-Alcalino en el Parque Natural de Cabo de Gata-Nijar (Almería). Estudio Volcanológico y Petrológico. Ph.D. Thesis, University of Granada, Granada, Spain, 1996; 295p. [Google Scholar]
- Rimstidt, J.D.; Balog, A.; Webb, J. Distribution of carbonate minerals and aqueous solutions. Geochim. Cosmochim. Acta 1998, 62, 1851–1863. [Google Scholar] [CrossRef]
- Anovitz, L.M.; Essene, E.J. Phase Equilibria in the System CaCO3-MgCO3-FeCO3. J. Petrol. 1987, 28, 389–415. [Google Scholar] [CrossRef]
- Heald, P.; Foley, N.K.; Hayba, D.O. Comparative anatomy of volcanic-hosted epithermal deposits; acid-sulfate and adularia-sericite types. Econ. Geol. 1987, 82, 1–26. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas., A.; Gonzalez-Urien, E. Exploration for epithermal gold deposits. In Gold in 2000; Hagemann, S.G., Brown, P.E., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2000. [Google Scholar]
- Wang, Y.; Xu, H. Prediction of trace metal partitioning between minerals and aqueous solutions: A linear free energy correlation approach. Geochim. Cosmochim. Acta 2001, 65, 1529–1543. [Google Scholar] [CrossRef]
- Böttcher, M.E.; Dietzel, M. Metal-ion partitioning during low-temperature precipitation and dissolution of anhydrous carbonates and sulphates. Eur. Mineral. Union Notes Mineral. 2010, 10. [Google Scholar] [CrossRef]
- Scott, S.D. Submarine hydrothermal systems and deposits. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 435–486. [Google Scholar]
- Yardley, B.W.D. Metal concentrations in crustal fluids and their relationship to ore formation. Econ. Geol. 2005, 100, 613–632. [Google Scholar] [CrossRef]
- McKibben, M.A.; Hardie, L.A. Ore-forming brines in active continental rifts. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 877–935. [Google Scholar]
- Charrier, R.; Ramos, V.A.; Tapia, F.; Sagripanti, L. Tectono-stratigraphic evolution of the Andean Orogen between 31 and 37°S (Chile and Western Argentina). Geol. Soc. Lond. Spec. Publ. 2015, 399. [Google Scholar] [CrossRef]
- Wilson, N.S.F.; Zentilli, M.; Reynolds, P.H.; Boric, R. Age of mineralization by basinal fluids at the El Soldado manto-type copper deposit, Chile: 40Ar/39Ar geochronology of K-feldspar. Chem. Geol. 2003, 197, 161–176. [Google Scholar] [CrossRef]











| Palai-Islica (Spain) | El Dorado (Chile) | Quebrada Marquesa (Chile) | Quebrada Marquesa (Chile) | Arqueros (Chile) | Melipilla (Chile) | |
|---|---|---|---|---|---|---|
| Deposit type | Epithermal Au-Cu volcanic-hosted | Epithermal Au-Cu volcanic-hosted | “Chilean Manto-type” Cu(-Ag) | Stratabound Mn | Ag-Ba epithermal veins | “Chilean Manto-type” Cu(-Ag) |
| Tectonic Setting | Extension after Alpine orogenesis | Compresional, arc setting | Extension, intra-arc basin | Extension, intra-arc basin | Extension, intra-arc basin | Extension, intra-arc basin |
| Morphology | Stockwork veins (and replacement) | Stockwork veins | Veins and replacement (stratabound) | Stratiform | Veins | Veins and replacement (stratabound) |
| Host rock | Andesites, dacites (Carboneras C-3 Formation) | Andesites (Los Elquinos Formation) | Andesites, basaltic andesites (Quebrada Marquesa Formation) | Andesites, basaltic andesites (Arqueros Formation and Quebrada Marquesa Formation) | Andesites, basaltic andesites (Arqueros Formation) | Andesites, basaltic andesites, limestones (Lo Prado and Veta Negra Formations) |
| Hydrothermal alteration | Pervasive (argillic to propylitic) | Pervasive (argillic to propylitic?) | Not well developed. Associated with very low-grade assemblages? | Not well developed. Associated with very low-grade assemblages | Not well developed. Associated/obliterated? by very low-grade assemblages | Not well developed. Associated with very low-grade assemblages |
| Ore Mineralogy | Pyrite, chalcopyrite ± sphalerite, galena (gold, Ag-bearing minerals) | Pyrite, chalcopyrite ± fahlore (gold) | Chalcopyrite, bornite ± sphalerite, galena, chalcocite (fahlore, stromeyerite) | Braunite, piemontite (pyrolusite) | Ag, Ag sulfide, fahlore, bornite, chalcocite | Chalcopyrite, bornite, pyrite ± chalcocite (fahlore, arsenopyrite...) |
| Gangue Mineralogy | Quartz, sericite, chlorite, kaolinite, pyrophyllite (dolomite(-ankerite), barite, siderite) | Quartz, sericite, kaolinite (ankerite) | Calcite, barite (celadonite) | Calcite, barite | Calcite, barite (chlorite) | Calcite, celadonite, quartz, chalcedony |
| Age | Tortonian | Upper Creteceous -Paleocene? | Albian-Cenomanian | Lower Cretaceous | Albian-Cenomanian? | Albian-Cenomanian |
| Location | ~1°55′ W Long., ~37°00′ N Lat. | ~70°44′ W Long., ~29°47′S Lat. | ~70°52′ W Long., ~29°54′ S Lat. | ~70°54′ W Long., ~29°54′ S Lat. | ~70°54′ W Long., ~29°51′ S Lat. | ~71°01′ W Long., ~33°49′ S Lat. |
| References | [23,24,25,26,27,28] | [29] | [25,30,31,32,33] | [25,30,31,32] | [25,30,31,32] | [33] |
| Mn (µMol/kg) | Ca (µMol/kg) | Mn at. % (Calcite) | |||
|---|---|---|---|---|---|
| 150 °C | 200 °C | 250 °C | |||
| Hydrothermal sea vents (1) | 15 | 22,000 | 0.11 | 0.07 | 0.04 |
| 6800 | 80,000 | 14.03 | 8.93 | 5.53 | |
| Geothermal fluids (2,3) | 0 | 7 | 0.42 | 0.27 | 0.17 |
| 146 | 699 | 25.00 | 21.89 | 13.55 | |
| Metamorphic fluids (2) | 0 | 136 | 0.00 | 0.00 | 0.00 |
| 5 | 475 | 1.74 | 1.10 | 0.68 | |
| Sea water (1) | 0 | 10,200 | 0.00 | 0.00 | 0.00 |
| Mn (at. %) Calcite | Mn (µMol/kg) 150 °C | Mn (µMol/kg) 200 °C | Mn (uMol/kg) 250 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | ||
| Quebrada Marquesa (“Manto-type" Cu(-Ag)) | 0.01 | 0 | 2 | 4 | 1 | 3 | 7 | 1 | 5 | 11 |
| 1.63 | 101 | 504 | 1008 | 158 | 792 | 1584 | 256 | 1280 | 2559 | |
| 0.51 | 31 | 156 | 313 | 49 | 246 | 491 | 79 | 397 | 793 | |
| Melipilla (“Manto-type" Cu(-Ag)) | 0.28 | 17 | 86 | 172 | 27 | 136 | 271 | 44 | 219 | 438 |
| 1.92 | 119 | 593 | 1186 | 186 | 932 | 1863 | 301 | 1505 | 3010 | |
| 0.65 | 40 | 200 | 400 | 63 | 314 | 628 | 101 | 507 | 1015 | |
| Quebrada Marquesa (Ag-Ba veins) | 0.03 | 2 | 10 | 20 | 3 | 16 | 31 | 5 | 25 | 50 |
| 0.94 | 58 | 291 | 583 | 92 | 458 | 916 | 148 | 740 | 1480 | |
| 0.37 | 23 | 115 | 230 | 36 | 180 | 361 | 58 | 291 | 583 | |
| Arqueros (stratabound Mn) | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.32 | 20 | 100 | 200 | 31 | 157 | 315 | 51 | 254 | 508 | |
| 0.04 | 3 | 13 | 26 | 4 | 21 | 42 | 7 | 34 | 67 | |
| Very low-grade metamorphism | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.08 | 5 | 26 | 52 | 8 | 41 | 82 | 13 | 66 | 132 | |
| 0.02 | 1 | 6 | 11 | 2 | 9 | 17 | 3 | 14 | 28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Rosúa, J.; Morales-Ruano, S.; Roberts, S.; Morata, D.; Belmar, M. Application of the Mineralogy and Mineral Chemistry of Carbonates as a Genetic Tool in the Hydrothermal Environment. Minerals 2021, 11, 822. https://doi.org/10.3390/min11080822
Carrillo-Rosúa J, Morales-Ruano S, Roberts S, Morata D, Belmar M. Application of the Mineralogy and Mineral Chemistry of Carbonates as a Genetic Tool in the Hydrothermal Environment. Minerals. 2021; 11(8):822. https://doi.org/10.3390/min11080822
Chicago/Turabian StyleCarrillo-Rosúa, Javier, Salvador Morales-Ruano, Stephen Roberts, Diego Morata, and Mauricio Belmar. 2021. "Application of the Mineralogy and Mineral Chemistry of Carbonates as a Genetic Tool in the Hydrothermal Environment" Minerals 11, no. 8: 822. https://doi.org/10.3390/min11080822
APA StyleCarrillo-Rosúa, J., Morales-Ruano, S., Roberts, S., Morata, D., & Belmar, M. (2021). Application of the Mineralogy and Mineral Chemistry of Carbonates as a Genetic Tool in the Hydrothermal Environment. Minerals, 11(8), 822. https://doi.org/10.3390/min11080822