The Influence of the Framework and Extraframework Content on the High Pressure Behavior of the GIS Type Zeolites: The Case of Amicite
Abstract
:1. Introduction
2. Amicite Structure
3. Experimental Methods
4. Results and Discussion
4.1. Amicite Compressed in Methanol:Ethanol:Water
4.2. Amicite Compressed in Silicone Oil
5. Comparison between Amicite Compressibility in Aqueous Medium and Silicone Oil
6. Compressibility Behavior of Microporous Materials with GIS Topology
- Compression of gismondine in both m.e.w. and s.o. favors the tetragonalization of the unit cell; in amicite the a and c axes also tend to become more similar at HP, but the beta angle does not substantially change;
- Gismondine compressed in m.e.w. undergoes a transition to a triclinic phase at about 3 GPa; the original symmetry of amicite, by contrast, is maintained in both the experiments;
- The HP framework deformation mechanism is the same in the two zeolites, essentially being driven by the distortion of the “double crankshaft” chains and the consequent change in the 8-ring channel shape;
- Amicite’s compressibility increases at HP both in m.e.w. and s.o.; by contrast, gismondine’s compressibility in s.o. slightly decreases while in m.e.w. it remains constant;
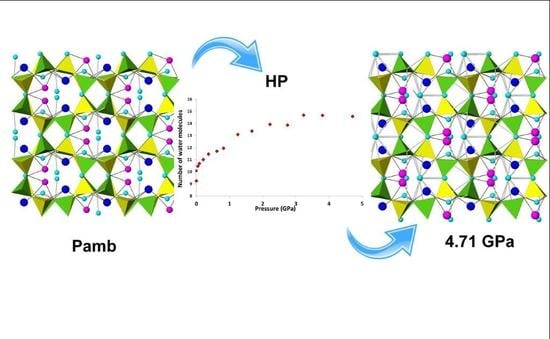
- PIH occurs in both amicite and in gismondine compressed in m.e.w. However, it induces different reorganizations in the water molecule systems: in amicite there is both the filling of partially occupied sites and the appearance of two new water sites; in gismondine four partially occupied water sites reduce to only two fully occupied sites, giving rise to a more ordered water system;
- In amicite 5.34 water molecules enter the zeolite porosities when compressed in m.e.w., while in gismondine only one additional molecule penetrates. This result can be explained by the higher channel stuffing of gismondine at Pamb compared to amicite;
- Both amicite and gismondine are more compressible in m.e.w. than in s.o., but for different reasons. In gismondine this effect has been justified by the re-organization of the water molecule system, which leaves a larger free volume inside the pores compared to the phase compressed in s.o. In amicite the higher compressibility at HP results from the strong bonds between framework oxygen atoms and the new water molecules;
- Overall, gismondine is more compressible than amicite, both in m.e.w. and in s.o. Comparing the unit cell volume decrease of the two phases at a similar pressure value—about 5.5 GPa—we find ΔV = −7.5% and −6.4% for gismondine in m.e.w. and s.o., respectively, while for amicite these values are −5.9% and −5.1%, respectively. The presence of the large potassium cations and the higher number of extraframework sites after PIH in amicite compared to gismondine probably contribute to better supporting the amicite structure.
- (a)
- The main feature of the P-induced evolution of cell parameters of K-GaSi-GIS is the noticeable squashing of the c axis, which is perpendicular to the dense plane and corresponds to the b axis of gismondine and amicite. This response to hydrostatic pressure corresponds to a gradual flattening of the double crankshaft chains and a reduction in the ellipticity of the 8-ring windows. The different behavior compared to amicite and gismondine, where the b axis slightly increases or remains almost unvaried, could be explained by the lower channel stuffing of the K-GaSi-GIS phase related to the high Si/Ga ratio;
- (b)
- In K-GaSi-GIS a PIH effect is again observed, with the penetration of about two water molecules at P < 1 GPa, but in this case the overhydration induces a disordering of the K-water system along the channels.
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gillet, P.; Malezieux, J.M.; Itie, J.P. Phase changes and amorphization of zeolites at high pressure: The case of scolecite and mesolite. Am. Mineral. 1996, 81, 651–657. [Google Scholar] [CrossRef]
- Huang, Y.; Havenga, E.A. Why do zeolites with LTA structure undergo reversible amorphization under pressure? Chem. Phys. Lett. 2001, 345, 65–71. [Google Scholar] [CrossRef]
- Rutter, M.D.; Uchida, T.; Secco, R.A.; Huang, Y.; Wang, Y. Investigation of pressure-induced amorphization in hydrated zeolite Li-A and Na-A using synchrotron X-ray diffraction. J. Phys. Chem. Solids 2001, 62, 599–606. [Google Scholar] [CrossRef]
- Greaves, G.N.; Meneau, F.; Sapelkin, A.; Colyer, L.M.; Gwynn, I.A.; Wade, S.; Sankar, G. The rheology of collapsing zeolites amorphized by temperature and pressure. Nat. Mater. 2003, 2, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Gulìn-González, J.; Suffritti, G.B. Amorphization of calcined LTA zeolites at high pressure: A computational study. Microporous Mesoporous Mater. 2004, 69, 127–134. [Google Scholar] [CrossRef]
- Goryainov, S.V. Pressure-induced amorphization of Na2Al2Si3O10·2H2O and KAlSi2O6 zeolites. Phys. Status Solidi 2005, 202, R25–R27. [Google Scholar] [CrossRef]
- Secco, R.A.; Huang, Y. Pressure-induced disorder in hydrated Na-A zeolite. J. Phys. Chem. Solids 1999, 60, 999–1002. [Google Scholar] [CrossRef]
- Rutter, M.D.; Secco, R.A.; Huang, Y. Ionic conduction in hydrated zeolite Li-, Na- and K-A at high pressures. Chem. Phys. Letter 2000, 331, 189–195. [Google Scholar] [CrossRef]
- Lee, Y.; Vogt, T.; Hriljac, J.A.; Parise, J.B.; Artioli, G. Pressure-induced volume expansion of zeolites in the natrolite family. J. Am. Chem. Soc. 2002, 124, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hriljac, J.A.; Vogt, T. Pressure-induced migration of zeolitic water in laumontite. Phys. Chem. Miner. 2004, 31, 421–428. [Google Scholar] [CrossRef]
- Ori, S.; Quartieri, S.; Vezzalini, G.; Dmitriev, V. Pressure-induced over-hydration and water ordering in gismondine: A synchrotron powder diffraction study. Am. Mineral. 2008, 93, 1393–1403. [Google Scholar] [CrossRef]
- Arletti, R.; Quartieri, S.; Vezzalini, G. Elastic behavior of zeolite boggsite in silicone oil and aqueous medium: A case of high-pressure-induced over-hydration. Am. Mineral. 2010, 95, 1247–1256. [Google Scholar] [CrossRef]
- Quartieri, S.; Montagna, G.; Arletti, R.; Vezzalini, G. Elastic behavior of MFI-type zeolites: Compressibility of H-ZSM-5 in penetrating and non-penetrating media. J. Solid State Chem. 2011, 184, 1505–1516. [Google Scholar] [CrossRef]
- Lotti, P.; Gatta, G.D.; Comboni, D.; Merlini, M.; Pastero, L.; Hanfland, M. AlPO4-5 zeolite at high pressure: Crystal–fluid interaction and elastic behavior. Microporous Mesoporous Mater. 2016, 228, 158–167. [Google Scholar] [CrossRef]
- Lee, Y.; Hriljac, J.A.; Vogt, T. Pressure-induced argon insertion into an auxetic small pore zeolite. J. Phys. Chem. C 2010, 114, 6922–6927. [Google Scholar] [CrossRef]
- Seoung, D.; Lee, Y.; Cynn, H.; Park, C.; Choi, K.Y.; Blom, D.A.; Evans, W.J.; Kao, C.C.; Vogt, T.; Lee, Y. Irreversible xenon insertion into a small-pore zeolite at moderate pressures and temperatures. Nat. Chem. 2014, 6, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Liu, D.; Seoung, D.; Liu, Z.; Kao, C.C.; Vogt, T. Pressure- and heat-induced insertion of CO2 into an auxetic small-pore zeolite. J. Am. Chem. Soc. 2011, 133, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Scelta, D.; Dziubek, K.; Ceppatelli, M.; Gorelli, F.A.; Bini, R.; Garbarino, G.; Thibaud, J.M.; Di Renzo, F.; Cambon, O.; et al. Synthesis of 1D polymer/zeolite nanocomposites under high pressure. Chem. Mater. 2016, 28, 4065–4071. [Google Scholar] [CrossRef]
- Gatta, G.D.; Lee, Y. Zeolites at high pressure: A review. Mineral. Mag. 2014, 78, 267–291. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Vezzalini, G.; Quartieri, S.; Alberti, A. Structural modifications induced by dehydration in the zeolite gismondine. Zeolites 1993, 13, 34–42. [Google Scholar] [CrossRef]
- Betti, C.; Fois, E.; Mazzucato, E.; Medici, C.; Quartieri, S.; Tabacchi, G.; Vezzalini, G.; Dmitriev, V. Gismondine under HP: Deformation mechanism and re-organization of the extra-framework species. Microporous Mesoporous Mater. 2007, 103, 190–209. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.J.; Kao, C.C.; Vogt, T. Pressure-induced hydration and order-disorder transition in a synthetic potassium gallosilicate zeolite with gismondine topology. J. Am. Chem. Soc. 2008, 130, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.N.; Kao, C.C.; Vogt, T.; Lee, Y. Anisotropic compression of a synthetic potassium aluminogermanate zeolite with gismondine topology. J. Solid State Chem. 2010, 183, 2305–2308. [Google Scholar] [CrossRef]
- Rocha, J.; Lin, Z. Micro and mesoporous mineral phases. Rev. Mineral. Geochem. 2005, 57, 173–201. [Google Scholar] [CrossRef]
- Danisi, R.M.; Armbruster, T.; Arletti, R.; Gatta, G.D.; Vezzalini, G.; Quartieri, S.; Dmitriev, V. Elastic behavior and pressure-induced structural modifications of the microporous Ca(VO)Si4O10∙4H2O dimorphs cavansite and pentagonite. Microporous Mesoporous Mater. 2015, 204, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the subcommittee on zeolites of the international mineralogical association, commission on new minerals and minerals names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Alberti, A.; Vezzalini, G. The crystal structure of amicite, a zeolite. Acta Cryst. 1979, B35, 2866–2869. [Google Scholar] [CrossRef]
- Miletich, R.; Allan, D.R.; Kush, W.F. High-temperature and high-pressure crystal chemistry. Rev. Mineral. Geochem. 2000, 41, 445–519. [Google Scholar] [CrossRef]
- Forman, R.A.; Piermarini, G.J.; Barnett, J.D.; Block, S. Pressure measurement made by the utilization of ruby sharp-line luminescence. Science 1972, 176, 4673–4676. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 9, 4673–4676. [Google Scholar] [CrossRef]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Häusermann, D. Two dimensional detector software: From real detector to idealized image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Larson, A.C.; von Dreele, R.B. GSAS-General Structure Analysis System; Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 1996. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Thomson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Cryst. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Colligan, M.; Forster, P.M.; Cheetham, A.K.; Lee, Y.; Vogt, T.; Hriljac, J.A. Synchrotron X-ray powder diffraction and computational investigation of purely siliceous zeolite Y under pressure. J. Am. Chem. Soc. 2004, 126, 12015–12022. [Google Scholar] [CrossRef] [PubMed]
- Likhacheva, A.Y.; Seryotkin, Y.V.; Manakov, A.Y.; Goryainov, S.V.; Ancharov, A.I.; Sheromov, M.A. Anomalous compression of scolecite and thomsonite in aqueous medium to 2 GPa. High Press. Res. 2006, 26, 449–453. [Google Scholar] [CrossRef]
- Likhacheva, A.Y.; Seryotkin, Y.V.; Manakov, A.Y.; Goryainov, S.V.; Ancharov, A.I.; Sheromov, M.A. Pressure-induced over-hydration of thomsonite: A synchrotron powder diffraction study. Am. Mineral. 2007, 92, 1610–1615. [Google Scholar] [CrossRef]






| P (GPa) | Pamb | 1.25 GPa | 4.71 GPa | Pamb(rev) |
|---|---|---|---|---|
| Space Group | I2 | I2 | I2 | I2 |
| a (Å) | 10.2324(8) | 10.1882(9) | 9.8661(5) | 10.2296(5) |
| b (Å) | 10.43456(8) | 10.443(1) | 10.4966(7) | 10.4328(6) |
| c (Å) | 9.8987(7) | 9.8651(8) | 9.6887(5) | 9.8931(4) |
| V (Å3) | 1056.63(2) | 1048.7(2) | 1002.59(8) | 1055.39(8) |
| β(°) | 88.382(6) | 87.49(9) | 87.728(8) | 88.349(6) |
| xRp (%) | 0.7 | 0.7 | 0.7 | 1.0 |
| Rwp (%) | 0.5 | 0.4 | 0.4 | 1.0 |
| R F2 (%) | 11.0 | 15.3 | 16.7 | 11.8 |
| No. of variables | 88 | 94 | 96 | 88 |
| P (GPa) | a (Å) | b (Å) | c (Å) | V (Å3) | β (°) |
|---|---|---|---|---|---|
| amicite (m.e.w.) | |||||
| Pamb | 10.2324(8) | 10.43456(8) | 9.8987(7) | 1056.63(2) | 88.382(6) |
| 0.04 | 10.2375(6) | 10.4383(6) | 9.8949(5) | 1056.96(1) | 88.343(5) |
| 0.09 | 10.2378(7) | 10.44088(7) | 9.8925(6) | 1056.96(2) | 88.294(5) |
| 0.21 | 10.241(8) | 10.4429(7) | 9.886(6) | 1056.81 | 88.170(5) |
| 0.36 | 10.244(6) | 10.44(1) | 9.8846(8) | 1057(1) | 88.03(7) |
| 0.62 | 10.2447(7) | 10.4365(7) | 9.8859(6) | 1056.4(2) | 87.891(6) |
| 0.82 | 10.2334(7) | 10.4289(8) | 9.8857(6) | 1054.3(2) | 87.786(7) |
| 1.25 | 10.1882(9) | 10.443(1) | 9.8651(8) | 1048.7(2) | 87.49(9) |
| 1.67 | 10.1290(9) | 10.471(1) | 9.8622(8) | 1044.7(2) | 87.236(7) |
| 2.22 | 10.073(1) | 10.485(1) | 9.853(1) | 1039.6(3) | 87.395(9) |
| 2.75 | 10.027(1) | 10.498(1) | 9.823(1) | 1033.1(3) | 87.52(1) |
| 3.23 | 9.9823(6) | 10.4975 | 9.7881(5) | 1024.78(9) | 87.588(9) |
| 3.80 | 9.9382(5) | 10.5004(6) | 9.7507(5) | 1016.66(8) | 87.633(8) |
| 4.71 | 9.8661(5) | 10.4966(7) | 9.6887(5) | 1002.59(8) | 87.728(8) |
| 5.35 | 9.820(3) | 10.500(4) | 9.648(3) | 994.1(9) | 87.86(3) |
| 6.29 | 9.747(4) | 10.503(5) | 9.588(4) | 981(1) | 87.95(3) |
| 6.71 | 9.717(4) | 10.505(5) | 9.568(4) | 976(1) | 88.08(4) |
| 6.91 | 9.693(5) | 10.502(5) | 9.551(4) | 972(1) | 88.18(4) |
| 7.48 | 9.657(5) | 10.500(6) | 9.526(5) | 966(1) | 88.24(5) |
| 8.13 | 9.618(6) | 10.498(7) | 9.496(6) | 958(1) | 88.24(6) |
| 6.45(rev) | 9.732(5) | 10.527(5) | 9.598(4) | 983(1) | 88.28(4) |
| 4.42(rev) | 9.894(2) | 10.528(3) | 9.726(2) | 1012.4(6) | 87.94(2) |
| 2.05(rev) | 10.108(1) | 10.481(1) | 9.884(1) | 1046.1(3) | 87.44(1) |
| Pamb(rev) | 10.2296(5) | 10.4328(6) | 9.8931(4) | 1055.39(8) | 88.349(6) |
| amicite (s.o.) | |||||
| Pamb | 10.2372(9) | 10.4352(9) | 9.892(8) | 1056.30(2) | 88.269(8) |
| 0.39 | 10.2271(6) | 10.4319(7) | 9.8686(5) | 1052.25(8) | 88.071(7) |
| 0.78 | 10.2133(8) | 10.4279(9) | 9.8528(7) | 1048.70(1) | 87.97(1) |
| 1.23 | 10.1923(9) | 10.434(1) | 9.8411(7) | 1045.90(1) | 87.890(1) |
| 1.72 | 10.160(1) | 10.452(2) | 9.828(1) | 1043(2) | 87.8(2) |
| 2.18 | 10.111(2) | 10.468(2) | 9.808(2) | 1037.4(2) | 87.720(3) |
| 2.89 | 10.026(3) | 10.505(4) | 9.779(8) | 1029.4(4) | 88.020(6) |
| 3.35 | 9.994(4) | 10.519(4) | 9.77(3) | 1026.6(4) | 88.16(6) |
| 3.86 | 9.9440(4) | 10.535(5) | 9.747(3) | 1020.7(5) | 88.38(7) |
| 4.27 | 9.911(4) | 10.541(5) | 9.729(3) | 1016.1(5) | 88.55(4) |
| 4.87 | 9.874(3) | 10.537(3) | 9.717(2) | 1010.5(5) | 88.3(4) |
| 5.48 | 9.816(6) | 10.555(6) | 9.682(5) | 1002.6(2) | 88.91(9) |
| Pamb(rev) | 10.239(6) | 10.432(7) | 9.8981(5) | 1056.8(2) | 88.26(9) |
| x/a | y/b | z/c | Occ | Uiso (*100) | |
|---|---|---|---|---|---|
| Pamb | |||||
| K | 0.324(1) | −0.014(2) | 0.960(1) | 1 | 0.91(3) |
| Na | 0.438(1) | 0.241(2) | 0.661(1) | 1 | 0.91(3) |
| Al1 | 0.153(2) | 0.241(3) | 0.150(2) | 1 | 0.6(3) |
| Al2 | 0.152(2) | –0.005(3) | 0.658(2) | 1 | 0.6(3) |
| Si1 | 0.156(2) | –0.023(3) | 0.327(2) | 1 | 0.6(3) |
| Si2 | 0.159(2) | 0.255(3) | 0.820(2) | 1 | 0.6(3) |
| O1 | 0.006(2) | –0.055(4) | 0.303(4) | 1 | 1.5(3) |
| O2 | –0.006(2) | 0.273(5) | 0.205(3) | 1 | 1.5(3) |
| O3 | 0.206(3) | 0.135(3) | 0.730(4) | 1 | 1.5(3) |
| O4 | 0.176(3) | 0.008(5) | 0.484(2) | 1 | 1.5(3) |
| O5 | 0.182(3) | 0.226(5) | 0.977(2) | 1 | 1.5(3) |
| O6 | 0.187(4) | 0.107(3) | 0.247(4) | 1 | 1.5(3) |
| O7 | 0.255(4) | 0.356(3) | 0.215(3) | 1 | 1.5(3) |
| O8 | 0.765(3) | 0.386(3) | 0.216(4) | 1 | 1.5(3) |
| Wat1 | 0.307(4) | 0.267(6) | 0.475(5) | 0.75(3) | 0.2(6) |
| Wat2 | 0.456(3) | 0.090(3) | 0.243(3) | 1 | 0.2(6) |
| Wat3 | 0 | 0.300(6) | 0.5 | 1 | 0.2(6) |
| Wat4 | 0.5 | 0.42(4) | 0.5 | 0.12(3) | 0.2(6) |
| 1.25 GPa | |||||
| K | 0.331(1) | -0.001(2) | 0.964(1) | 1 | 4.5(4) |
| Na | 0.449(2) | 0.276(3) | 0.672(2) | 1 | 4.5(4) |
| Al1 | 0.143(1) | 0.249(2) | 0.147(2) | 1 | 0.4(2) |
| Al2 | 0.164(2) | 0.007(2) | 0.659(2) | 1 | 0.4(2) |
| Si1 | 0.151(1) | –0.010(2) | 0.331(2) | 1 | 0.4(2) |
| Si2 | 0.159(1) | 0.265(3) | 0.822(2) | 1 | 0.4(2) |
| O1 | –0.003(1) | –0.031(3) | 0.301(3) | 1 | 0.7(3) |
| O2 | –0.008(1) | 0.307(3) | 0.201(3) | 1 | 0.7(3) |
| O3 | 0.218(2) | 0.146(3) | 0.730(4) | 1 | 0.7(3) |
| O4 | 0.182(3) | 0.028(4) | 0.486(1) | 1 | 0.7(3) |
| O5 | 0.183(3) | 0.230(4) | 0.977(2) | 1 | 0.7(3) |
| O6 | 0.183(3) | 0.117(3) | 0.241(3) | 1 | 0.7(3) |
| O7 | 0.256(3) | 0.365(3) | 0.185(3) | 1 | 0.7(3) |
| O8 | 0.756(3) | 0.389(3) | 0.230(2) | 1 | 0.7(3) |
| Wat1 | 0.283(2) | 0.257(5) | 0.467(3) | 1 | 1.7(5) |
| Wat2 | 0.480(3) | 0.105(4) | 0.277(3) | 1 | 1.7(5) |
| Wat3 | 0 | 0.259(6) | 0.5 | 1 | 1.7(5) |
| Wat4 | 0.5 | 0.459(7) | 0.5 | 0.74(3) | 1.7(5) |
| Wat5 | 0.570(6) | 0.172(5) | 0.964(4) | 0.40(3) | 1.7(5) |
| 4.71 GPa | |||||
| K | 0.340(1) | 0.006(2) | 0.965(2) | 1 | 5.1(5) |
| Na | 0.448(2) | 0.276(3) | 0.672(2) | 1 | 5.1(5) |
| Al1 | 0.164(2) | 0.259(2) | 0.154(2) | 1 | 2.1(3) |
| Al2 | 0.166(2) | 0.024(3) | 0.657(2) | 1 | 2.1(3) |
| Si1 | 0.161(2) | 0.009(2) | 0.32(2) | 1 | 2.1(3) |
| Si2 | 0.152(2) | 0.275(3) | 0.822(2) | 1 | 2.1(3) |
| O1 | 0.001(2) | −0.009(4) | 0.303(3) | 1 | 2.4(4) |
| O2 | 0.004(2) | 0.316(4) | 0.199(4) | 1 | 2.4(4) |
| O3 | 0.220(3) | 0.167(3) | 0.723(3) | 1 | 2.4(4) |
| O4 | 0.206(3) | 0.044(4) | 0.483(2) | 1 | 2.4(4) |
| O5 | 0.174(3) | 0.229(3) | 0.980(2) | 1 | 2.4(4) |
| O6 | 0.216(3) | 0.143(3) | 0.265(3) | 1 | 2.4(4) |
| O7 | 0.225(3) | 0.403(3) | 0.209(3) | 1 | 2.4(4) |
| O8 | 0.766(3) | 0.404(3) | 0.218(4) | 1 | 2.4(4) |
| Wat1 | 0.290(2) | 0.259(5) | 0.472(4) | 1 | 2.5(5) |
| Wat2 | 0.494(3) | 0.078(3) | 0.247(3) | 1 | 2.5(5) |
| Wat3 | 0 | 0.301(1) | 0.5 | 0.80(3) | 2.5(5) |
| Wat4 | 0.5 | 0.494(7) | 0.5 | 1 | 2.5(5) |
| Wat5 | 0.5 | 0.234(7) | 0 | 1 | 2.5(5) |
| Wat6 | 0 | 0.590(10) | 0 | 0.48(3) | 2.5(5) |
| Pamb(rev) | |||||
| K | 0.335(1) | 0.002(2) | 0.967(1) | 1 | 3.0(4) |
| Na | 0.446(2) | 0.270(3) | 0.671(2) | 1 | 3.0(4) |
| Al1 | 0.143(1) | 0.256(2) | 0.154(2) | 1 | 0.3(2) |
| Al2 | 0.164(2) | 0.017(3) | 0.651(2) | 1 | 0.3(2) |
| Si1 | 0.153(1) | −0.012(2) | 0.323(2) | 1 | 0.3(2) |
| Si2 | 0.166(1) | 0.269(2) | 0.824(2) | 1 | 0.3(2) |
| O1 | –0.004(1) | −0.017(3) | 0.303(3) | 1 | 0.4(4) |
| O2 | –0.013(1) | 0.304(3) | 0.198(3) | 1 | 0.4(4) |
| O3 | 0.220(3) | 0.151(3) | 0.732(4) | 1 | 0.4(4) |
| O4 | 0.186(3) | 0.027(4) | 0.478(2) | 1 | 0.4(4) |
| O5 | 0.174(4) | 0.233(4) | 0.983(2) | 1 | 0.4(4) |
| O6 | 0.183(3) | 0.121(3) | 0.244(4) | 1 | 0.4(4) |
| O7 | 0.244(3) | 0.374(3) | 0.213(3) | 1 | 0.4(4) |
| O8 | 0.767(3) | 0.405(3) | 0.218(3) | 1 | 0.4(4) |
| Wat1 | 0.278(2) | 0.271(5) | 0.473(3) | 0.92(2) | 0.2(7) |
| Wat2 | 0.449(3) | 0.104(2) | 0.241(3) | 1 | 0.2(7) |
| Wat3 | 0 | 0.329(5) | 0.5 | 1 | 0.2(7) |
| Wat4 | 0.5 | 0.457(3) | 0.5 | 0.16(3) | 0.2(7) |
| Pamb | 1.25 GPa | 4.71 GPa | Pamb(rev) | ||
|---|---|---|---|---|---|
| Al1- | O2 | 1.732(3) | 1.721(2) | 1.721(3) | 1.721(3) |
| O5 | 1.738(3) | 1.722(2) | 1.721(3) | 1.727(3) | |
| O6 | 1.732(3) | 1.720(2) | 1.719(3) | 1.720(3) | |
| O7 | 1.732(3) | 1.720(2) | 1.719(3) | 1.721(3) | |
| Al2- | O1 | 1.733(3) | 1.721(2) | 1.720(3) | 1.723(3) |
| O3 | 1.731(3) | 1.721(2) | 1.720(3) | 1.721(3) | |
| O4 | 1.735(3) | 1.721(2) | 1.720(3) | 1.724(3) | |
| O8 | 1.731(3) | 1.720(2) | 1.720(3) | 1.719(3) | |
| Si1- | O1 | 1.603(3) | 1.620(2) | 1.620(3) | 1.622(3) |
| O4 | 1.606(3) | 1.620(2) | 1.620(3) | 1.624(3) | |
| O6 | 1.601(3) | 1.620(2) | 1.619(3) | 1.620(3) | |
| O7 | 1.601(3) | 1.620(2) | 1.619(3) | 1.620(3) | |
| Si2- | O2 | 1.602(3) | 1.6202(2) | 1.620(3) | 1.622(3) |
| O3 | 1.601(3) | 1.6203(2) | 1.620(3) | 1.621(3) | |
| O5 | 1.608(3) | 1.6211(2) | 1.621(3) | 1.626(3) | |
| O8 | 1.600(3) | 1.6196(2) | 1.620(3) | 1.620(3) | |
| K- | O3 | 3.07(1) | 3.06(2) | 3.11(2) | 3.06(1) |
| O5 | 2.89(2) | 2.78(2) | 2.82(1) | 2.92(2) | |
| O8 | 2.75(1) | 2.76(2) | 2.77(1) | 2.78(1) | |
| Wat1 | 2.71(4) | 2.86(4) | 2.94(1) | 2.73(4) | |
| Wat2 | 3.19(2) | 3.19(3) | 2.69(2) | 3.16(3) | |
| Wat3 | 2.67(4) | 3.07(5) | 2.69(1) | 3.16(2) | |
| Wat5 | 3.20(7) | 2.85(1) | 2.50(4) | ||
| Wat5 | 2.28(8) | 2.85(1) | |||
| Na- | O1 | 2.65(2) | 2.38(2) | 2.74(1) | 2.63(3) |
| O3 | 2.68(1) | 2.77(2) | 2.52(1) | 2.68(2) | |
| O8 | 2.822) | 2.52(2) | 2.68(2) | 2.79(2) | |
| Wat1 | 2.33(5) | 2.70(4) | 2.50(2) | 2.65(3) | |
| Wat1 | 2.92(3) | 3.00(2) | 2.98(2) | 3.13(3) | |
| Wat2 | 2.15(3) | 2.10(5) | 2.29(1) | 2.23(3) | |
| Wat4 | 2.52(3) | 2.59(5) | 2.86(1) | 2.62(2) | |
| Wat5 | |||||
| Wat6 | 2.54(1) | ||||
| Wat1- | K | 2.86(4) | 2.94(1) | 2.73(4) | |
| Na | 2.33(5) | 2.70(4) | 2.50(2) | 2.65(3) | |
| Na | 2.92(3) | 3.00(2) | 2.98(2) | 3.13(3) | |
| O3 | 3.02(4) | 2.88(3) | 2.63(1) | 2.90(3) | |
| O4 | 3.01(6) | 2.61(5) | 2.37(1) | 2.70(5) | |
| O6 | 3.09(6) | 2.82(4) | 2.48(1) | 2.93(4) | |
| O7 | 2.79(5) | 3.03(3) | 3.05(1) | 2.82(3) | |
| Wat2 | 3.12(5) | ||||
| Wat3 | 3.16(4) | 2.89(2) | 2.84(1) | 2.91(2) | |
| Wat4 | 2.55(3) | 3.08(6) | 3.01(2) | ||
| Wat6 | 2.75(1) | ||||
| Wat2- | K | 3.191(2) | 3.19(3) | 2.69(2) | 3.16(3) |
| K | 3.16(2) | ||||
| Na | 2.152(3) | 2.10(5) | 2.29(1) | 2.23(3) | |
| O2 | 3.13(4) | 2.78(2) | |||
| O3 | 3.11(4) | 3.02(1) | |||
| O6 | 2.752(3) | 3.07(4) | 2.77(1) | 2.73(3) | |
| O7 | 2.83(1) | 3.14(2) | |||
| O8 | 3.081) | ||||
| Wat1 | 3.12(4) | ||||
| Wat5 | 2.556(3) | 2.911) | |||
| Wat6 | 2.42(1) | ||||
| Wat3- | K(x2) | 2.67(4) | 3.07(5) | 2.69(1) | 2.50(4) |
| O2(x2) | 2.939(6) | 3.054(9) | 2.911) | 3.009(4) | |
| O4 (x2) | 3.02(5) | ||||
| Wat1(x2) | 3.16(4) | 2.89(2) | 2.84(1) | 2.911(2) | |
| Wat4- | Na(x2) | 2.52(3) | 2.59(5) | 2.86(1) | 2.62(2) |
| O1(x2) | 3.01(4) | 2.973(7) | 2.93(1) | 3.008(3) | |
| Wat1 (x2) | 3.08(6) | 2.96(1) | 3.01(2) | ||
| Wat5- | K | 3.03(6) | 2.85(1) | ||
| K | 2.19(8) | 2.85(1) | |||
| Na | 3.03(8) | ||||
| O5 | 2.67(8) | ||||
| O6 | 3.20(6) | ||||
| O7 | 3.01(7) | ||||
| Wat2 | 2.556(3) | 2.91(1) | |||
| Wat2 | 2.91(1) | ||||
| Wat6- | Na (x2) | 2.54(1) | |||
| O4 (x2) | 2.97(2) | ||||
| Wat1 (x2) | 2.75(1) | ||||
| Wat2 (x2) | 2.42(1) | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arletti, R.; Giacobbe, C.; Quartieri, S.; Vezzalini, G. The Influence of the Framework and Extraframework Content on the High Pressure Behavior of the GIS Type Zeolites: The Case of Amicite. Minerals 2017, 7, 18. https://doi.org/10.3390/min7020018
Arletti R, Giacobbe C, Quartieri S, Vezzalini G. The Influence of the Framework and Extraframework Content on the High Pressure Behavior of the GIS Type Zeolites: The Case of Amicite. Minerals. 2017; 7(2):18. https://doi.org/10.3390/min7020018
Chicago/Turabian StyleArletti, Rossella, Carlotta Giacobbe, Simona Quartieri, and Giovanna Vezzalini. 2017. "The Influence of the Framework and Extraframework Content on the High Pressure Behavior of the GIS Type Zeolites: The Case of Amicite" Minerals 7, no. 2: 18. https://doi.org/10.3390/min7020018
APA StyleArletti, R., Giacobbe, C., Quartieri, S., & Vezzalini, G. (2017). The Influence of the Framework and Extraframework Content on the High Pressure Behavior of the GIS Type Zeolites: The Case of Amicite. Minerals, 7(2), 18. https://doi.org/10.3390/min7020018