The Petrography, Mineralogy and Geochemistry of Some Cu- and Pb-Enriched Coals from Jungar Coalfield, Northwestern China
Abstract
:1. Introduction
2. Geological Setting of the Jungar Coalfield
3. Samples and Analytical Procedures
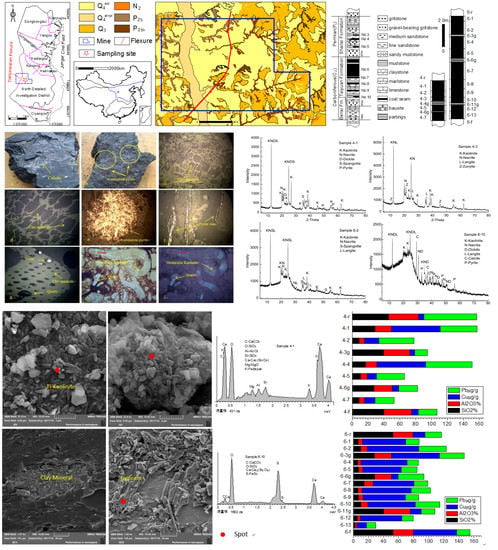
4. Results
4.1. Coal Petrology and Vitrinite Reflectance
4.2. Coal Chemistry
4.3. Coal Geochemistry
4.3.1. Major Elements
4.3.2. Trace Elements
Grouping of Coal Bench Samples Based on the Coal Forming Environment
Variations in Trace Elements through Vertical Coal Seam Sections
Distribution Pattern of Rare Earth Elements and Yttrium
4.4. Minerals in Coals and Host Rocks
5. Discussion
5.1. Cu and Pb in Jungar Coal
5.2. Causes of Enrichment
5.3. Occurrence
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, T.H.; Huang, W.H.; Yan, D.Y.; Tang, X.Y. Progress of research on mineralization mode of large coal-Ge deposits in China: Coal-Ge deposit in Wulantuga of Inner Mongolia and Lincang of Yunan. Earth Sci. Front. 2016, 23, 113–123. (In Chinese) [Google Scholar]
- Dai, S.F.; Chekryzhov, I.Y.; Seredin, V.V.; Nechaev, V.P.; Graham, I.T.; Hower, J.C.; Ward, C.R.; Ren, D.Y.; Wang, X.B. Metalliferous coal deposits in East Asia (Primorye of Russia and South China): A review of geodynamic controls and styles of mineralization. Gondwana Res. 2016, 29, 60–82. [Google Scholar] [CrossRef]
- Dai, S.F.; Liu, J.J.; Ward, C.R.; Hower, J.C.; Xie, P.P.; Jiang, Y.F.; Hood, M.M.; O’Keefe, J.M.K.; Song, H.J. Petrological, geochemical, and mineralogical compositions of the low-Ge coals from the Shengli coalfield, China: A comparative study with Ge-rich coals and a formation model for coal-hosted Ge ore deposit. Ore Geol. Rev. 2015, 71, 318–349. [Google Scholar] [CrossRef]
- Dai, S.F.; Liu, J.J.; Ward, C.R.; Hower, J.C.; French, D.; Jia, S.H.; Hood, M.M.; Garrison, T.M. Mineralogical and geochemical compositions of Late Permian coals and host rocks from the Guxu coalfield, Sichuan Province, China, with emphasis on enrichment of rare metals. Int. J. Coal Geol. 2016, 166, 71–95. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.; Henke, K.; Hood, M.; Eble, C.; Honaker, R.; Qian, D. Notes on the potential for the concentration of rare earth elements and Yttrium in coal combustion fly ash. Minerals 2015, 5, 356–366. [Google Scholar] [CrossRef]
- Marshall, C.; Large, D.J.; Meredith, W.; Snape, C.E.; Uguna, C.; Spiro, B.F.; Orheim, A.; Jochmann, M.; Mokogwu, I.; Wang, Y.; et al. Geochemistry and petrology of Palaeocene coals from Spitsbergen—Part 1: Oil potential and depositional environment. Int. J. Coal Geol. 2015, 143, 22–33. [Google Scholar] [CrossRef]
- Dai, S.F.; Yang, J.Y.; Ward, C.R.; Hower, J.C.; Liu, H.D.; Garrison, T.M.; French, D.; O’Keefe, J.M.K. Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin, Xinjiang, northwestern China. Ore Geol. Rev. 2015, 70, 1–30. [Google Scholar] [CrossRef]
- Dai, S.F.; Hower, J.C.; Ward, C.R.; Guo, W.; Song, H.J.; O’Keefe, J.M.K.; Xie, P.P.; Hood, M.M.; Yan, X.Y. Elements and phosphorus minerals in the middle Jurassic inertinite-rich coals of the Muli coalfield on the Tibetan Plateau. Int. J. Coal Geol. 2015, 144–145, 23–47. [Google Scholar] [CrossRef]
- Dai, S.; Seredin, V.; Ward, C.R.; Hower, J.C.; Xing, R.; Zhang, J.; Wang, Y. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding coalfield, Guizhou, China. Miner. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Zhao, C.L.; Li, Y.H.; Wang, J.X. Minimum mining grade of the selected trace elements in Chinese coal. J. China Coal Soc. 2014, 39, 744–748. (In Chinese) [Google Scholar] [CrossRef]
- Dai, S.F.; Wang, X.B.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.H.; Li, T.; Li, X.; Liu, H.D.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90–91, 72–99. [Google Scholar] [CrossRef]
- Tang, Y.G.; Chang, C.X.; Zhang, Y.Z. Migration and distribution of fifteen toxic trace elements during the coal washing of the Kailuan Coalfield, Hebei Province. Geochemical 2005, 4, 366–372. (In Chinese) [Google Scholar] [CrossRef]
- Song, D.Y.; Zhang, X.K.; Zhang, J.Y.; Zheng, C.G. Migration characteristics of hazardous trace elements in coal in the process of floatation. J. China Coal Soc. 2010, 7, 1170–1176. (In Chinese) [Google Scholar]
- Wang, W.F.; Qin, Y.; Song, D.Y. Study on the mobility and release of trace elements in coal-fired power plant. Acta Sci. Circumst. 2003, 23, 748–752. (In Chinese) [Google Scholar]
- Huang, J.M.; Zhang, J.Y.; Tian, C.; Zhang, S.B.; Zhao, Y.C.; Zheng, Y.C. Investigation on the transfer-transformation behavior of beryllium during coal combustion. J. Fuel Chem. Technol. 2016, 44, 648–653. (In Chinese) [Google Scholar]
- Yang, J.Y. Re-exploration on the law of trace elements migration during the pyrolysis of coal. J. China Coal Soc. 2013, 38, 2227–2233. (In Chinese) [Google Scholar]
- Swaine, D.J.; Fari, G. Environmental Aspects of Trace Elements in Coal, 1st ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 5–23. ISBN 90-481-4606-2. [Google Scholar]
- Zhao, F.H.; Ren, D.Y.; Zhang, J.Y.; Dai, S.F. Recent advance of study on hazards in coal and significance for environment protection. Coalmine Environ. Prot. 1998, 2, 20–23. (In Chinese) [Google Scholar]
- Tang, Y.G.; Zhang, H.Y.; Dai, S.F.; Yang, X.H.; Chen, D.Y. Geochemical characteristics of lead in coal. Coal Geol. Explor. 2001, 2, 7–10. (In Chinese) [Google Scholar]
- Wu, Y.Y.; Qin, Y.; Yi, T.S.; Xia, X.H. Enrichment and geochemical origin of some trace elements in high-sulfur coal from Kaili, eastern Guizhou Province. Geochemica 2008, 37, 615–622. (In Chinese) [Google Scholar]
- Yang, X.Y.; Liu, X.Z.; Du, X.; Wang, Y.H. Distribution of heavy metal element of coal powder in Jiangxi. Environ. Sci. Technol. 2009, 32, 115–117. (In Chinese) [Google Scholar]
- Zhu, C.S.; Li, D.H. Occurrences of trace elements in the No. 2 coal of the Changhebian coal mine, Chongqing, China. Bull. Miner. Petrol. Chem. 2009, 28, 259–263. (In Chinese) [Google Scholar]
- Cheng, W.; Yang, R.D.; Zhang, Q.; Cui, Y.C.; Gao, J.B. Distribution characteristics, occurrence modes and controlling factors of trace elements in Late Permian coal from Bijie City, Guizhou Province. J. China Coal Soc. 2013, 38, 103–113. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.B.; Trush, M.; Yager, J. DNA damage caused by reactive oxygen species originating from a copper-dependent oxidation of the 2-hydroxy catechol of estradiol. Carcinogenesis 1994, 15, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; John, M.H. Endothelial cell injury due to copper-catalyzed hydrogen peroxide generation from homocysteine. J. Clin. Investig. 1986, 77, 1370–1376. [Google Scholar] [CrossRef]
- Cornell University, Pesticide Information Profile for Copper Sulfate (May 1994). Available online: http://pmep.cce.cornell.edu/profiles/extoxnet/carbaryl-dicrotophos/copper-sulfate-ext.html (accessed on 10 July 2008).
- Brewer, G.J. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors (Oxf. Engl.) 2012, 38, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, J. Possible Role for Environmental Copper in Alzheimer’s Disease. Science 2003, 301, 905. [Google Scholar] [CrossRef] [PubMed]
- Swaine, D.J. Why trace elements are important. Fuel Process. Technol. 2000, 65–66, 21–33. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for carbonaceous biolithes: World average for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Gayer, R.A.; Rose, M.; Dehmer, J.; Shao, L.-Y. Impact of sulphur and trace elements geochemistry on the utilization of a marine-influenced coal-case study from the South Wales Variscan foreland basin. Int. J. Coal Geol. 1999, 40, 151–174. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Liu, J.R.; Li, S.S. Occurrence and distribution of minor toxic elements in coals of Fengfeng coalfield, Heibei Province, North China. J. China Univ. Min. Technol. 2003, 4, 20–24. (In Chinese) [Google Scholar]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Zhuang, X.G.; Gong, J.Q.; Wang, Z.Q.; Zeng, R.S.; Xu, W.D. Trace elements of the Late Permian coal in the Shuicheng and Liuzhi coal fields, Guizhou. Geol. Sci. Technol. Inf. 2001, 20, 53–58. (In Chinese) [Google Scholar]
- Ren, D.Y.; Xu, D.W.; Zhang, J.Y.; Zhao, F.H. Distribution of associated elements in coals from Shenbei coalfield. J. China Univ. Min. Technol. 1999, 28, 12–15. (In Chinese) [Google Scholar]
- Spears, D.A. Role of seawater on the trace element geochemistry of some UK coals and a tribute to Goldschmidt. Minerals 2017, 7, 300–314. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Shao, L.Y.; Zhao, M.J. Variation of coal geochemistry and special textures of late Permian coals in the western Guizhou province and their volcanic origin. Geochemica 2003, 3, 239–247. (In Chinese) [Google Scholar]
- Dai, S.F.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D.Y.; Ma, Y.W.; Sun, Y.Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Li, S.S.; Jiang, Y.F. Mineralogy and geochemistry of the No. 6 coal (Pennsylvanian) in the Jungar coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Wang, W.F.; Qin, Y.; Liu, X.H.; Zhao, J.L.; Wang, J.Y.; Wu, G.D.; Liu, J.T. Distribution, occurrence and enrichment causes of gallium in coals from the Jungar coalfield, Inner Mongolia. Sci. China Earth Sci. 2011, 41, 181–196. (In Chinese) [Google Scholar] [CrossRef]
- Lin, M.Y.; Chu, G.C.; Zhao, B.; Zhao, S.S.; Zhou, J.F.; Liu, B.J.; Shi, J. The coal petrology and trace element character for the No. 6 coal of Haerwusu coal mine. J. Jilin Univ. (Earth Sci. Ed.) 2015, 45, 1008. (In Chinese) [Google Scholar]
- Zhang, Y.H.; Wang, X.M.; Liu, D.N. Geochemical characteristics and geological significance of coal seam parting, roof and floor rare earth element in Southwestern Jungar Coalfield. China Coal Geol. 2014, 25, 13–16, 32. (In Chinese) [Google Scholar] [CrossRef]
- International Organization for Standardization. Methods for the Petrographic Analysis of Coals. Method of Determining Microscopically the Reflectance of Vitrinite; International Organization for Standardization: Geneva, Switzerland, 2009; ISBN 978-0-580-56505-2. [Google Scholar]
- International Committee for Coal and Organic Petrology (ICCP). The new vitrinite classification (ICCP System 1994). Fuel 1998, 77, 349–358. [Google Scholar]
- International Committee for Coal and Organic Petrology (ICCP). The new inertinite classification (ICCP System 1994). Fuel 2001, 80, 459–471. [Google Scholar]
- Dai, S.F.; Wang, X.B.; Zhou, Y.P.; Hower, J.C.; Li, D.H.; Chen, W.M.; Zhu, X.W.; Zou, J.H. Chemical and mineralogical compositions of silicic, mafic, and alkali tonsteins in the late Permian coals from the Songzao Coalfield, Chongqing, Southwest China. Chem. Geol. 2011, 282, 29–44. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.F. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution, 1st ed.; Blackwell: London, UK, 1985; p. 312. ISBN 90-481-4606-2. [Google Scholar]
- Li, H.; Chen, Y.Q.; Gui, G.F. Study of some environmental signification trace elements in coals from Bijie. J. Bijie Univ. 2009, 27, 77–79. (In Chinese) [Google Scholar]
- Wang, D.L.; Zhang, Y.H. Jurassic Tariqik Formation coal geochemical characteristics in Kuqa-Bay coalfield, Xinjiang. Coal Geol. China 2012, 24, 6–9. (In Chinese) [Google Scholar]
- Yang, L.; Liu, C.Y.; Li, H.Y. Geochemistry of trace elements and rare earth elements of coal in Chenjiashan coal mine. Coal Geology and Exploration 2008, 36, 10–14. (In Chinese) [Google Scholar]
- Lu, X.W.; Ge, K.L.; Wang, L.Z.; Wang, W.Y. The content of trace elements of coals in Weibei area, Shaanxi Province. J. Jilin Univ. (Earth Sci. Ed.) 2003, 2, 178–182. (In Chinese) [Google Scholar] [CrossRef]
- Pang, Q.F.; Zhuang, X.G.; Li, J.F.; Fu, L.M.; Gang, T.M.E.; Xu, Y. Petrographical, chemical and geochemical characteristics of Jurassic coal in Western Chaoshui basin, Inner Mongolia. Geol. Sci. Technol. Inf. 2012, 31, 27–32. (In Chinese) [Google Scholar]
- Zou, J.H.; Liu, D.; Tian, H.M.; Liu, F.; Li, T.; Yang, H.Y. Geochemistry of trace and rare earth elements in the Late Paleozoic coal from Adaohai mine, Inner Mongolia. J. China Coal Soc. 2013, 38, 1012–1018. (In Chinese) [Google Scholar] [CrossRef]
- Yang, M.D. Coal Mine Geology, 1st ed.; Coal Industry Press: Beijing, China, 2000; p. 159. ISBN 75-02-0191-03. (In Chinese) [Google Scholar]














| Sample | Mad | Ad | Vdaf | Fc,d | St,d | Sp,d | So,d | Ss,d | Vitrinite | Inertinite | Liptinite | Rr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-r | 0.92 | 86.14 | 13.81 | 0.05 | 0.1 | nd | nd | nd | nd | nd | nd | nd |
| 4-1 | 2.42 | 50.41 | 40.83 | 29.34 | 1.07 | 0.94 | 0.13 | 0 | 25.00 | 71.81 | 3.19 | nd |
| 4-2 | 4.18 | 17.47 | 38.37 | 50.87 | 0.42 | 0.14 | 0.28 | 0 | 50.00 | 47.09 | 2.91 | 0.58 |
| 4-3 | 4.45 | 31.92 | 38.88 | 41.61 | 0.33 | 0.07 | 0.26 | 0 | 29.03 | 68.71 | 2.26 | nd |
| 4-4 g | 1.94 | 72.36 | 15.89 | 11.75 | 0.06 | nd | nd | nd | nd | nd | nd | nd |
| 4-5 | 4.82 | 20.17 | 37.8 | 49.65 | 0.38 | 0.06 | 0.32 | 0 | 49.83 | 49.49 | 0.68 | nd |
| 4-6 g | 3.32 | 49.13 | 20.89 | 29.98 | 0.21 | nd | nd | nd | nd | nd | nd | nd |
| 4-7 | 4.32 | 18.79 | 34.92 | 52.85 | 1.28 | 0.97 | 0.30 | 0.01 | 46.80 | 52.10 | 1.10 | nd |
| 4-f | 1.71 | 77.82 | 16.27 | 5.91 | 0.14 | nd | nd | nd | nd | nd | nd | nd |
| Avg. Coal | 4.04 | 27.75 | 38.2 | 44.87 | 0.70 | 0.44 | 0.26 | 0 | 40.11 | 57.83 | 2.06 | nd |
| 6-r | 0.94 | 83.71 | 12.17 | 4.12 | 0.87 | nd | nd | nd | nd | nd | nd | nd |
| 6-1 | 3.86 | 13.52 | 36.9 | 54.57 | 0.74 | 0.28 | 0.46 | 0 | 65.14 | 24.77 | 10.09 | nd |
| 6-2 | 3.38 | 19.71 | 36.79 | 50.76 | 0.67 | 0.31 | 0.35 | 0 | 69.01 | 22.26 | 8.73 | nd |
| 6-3 g | 2.88 | 51.44 | 21.99 | 26.57 | 0.25 | nd | nd | nd | nd | nd | nd | nd |
| 6-4 | 4.16 | 14.38 | 39.36 | 51.92 | 0.52 | 0.13 | 0.39 | 0 | 61.41 | 30.43 | 8.16 | nd |
| 6-5 | 4.38 | 14.08 | 41.35 | 50.4 | 0.56 | 0.1 | 0.46 | 0 | 58.98 | 29.16 | 11.86 | nd |
| 6-6 g | 2.38 | 51.28 | 21.61 | 27.11 | 0.22 | nd | nd | nd | nd | nd | nd | nd |
| 6-7 | 3.28 | 26.84 | 38.68 | 44.86 | 0.51 | 0.12 | 0.39 | 0 | 53.13 | 33.75 | 13.13 | nd |
| 6-8 | 4.28 | 12.1 | 36.65 | 55.68 | 0.77 | 0.29 | 0.47 | 0.01 | 60.36 | 27.25 | 12.39 | nd |
| 6-9 | 3.83 | 17.06 | 40.4 | 49.43 | 0.71 | 0.21 | 0.5 | 0 | 64.58 | 24.67 | 10.75 | nd |
| 6-10 | 3.55 | 18.06 | 32.24 | 54.91 | 0.35 | 0.02 | 0.33 | 0 | 64.24 | 23.02 | 12.74 | 0.59 |
| 6-11 g | 1.56 | 77.45 | 15.62 | 6.93 | 0.06 | nd | nd | nd | nd | nd | nd | nd |
| 6-12 | 4.69 | 10.79 | 38.46 | 54.87 | 0.67 | 0.2 | 0.47 | 0 | 75.08 | 17.25 | 7.68 | nd |
| 6-13 | 3.96 | 11.22 | 38.19 | 52.29 | 1.48 | 1.02 | 0.44 | 0.02 | 68.94 | 20.08 | 10.97 | nd |
| 6-f | 1.14 | 81.21 | 13.29 | 5.5 | 0.09 | nd | nd | nd | nd | nd | nd | nd |
| Avg. Coal | 3.94 | 15.78 | 37.90 | 51.97 | 0.70 | 0.27 | 0.43 | 0.00 | 64.13 | 25.22 | 10.65 | nd |
| Sample | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | MnO | TiO2 | P2O5 | LOI | C | Si/Al | Sb | Li | Bi | Pb | Mo | U | Th | Cu | Zn | W | Ga | Sr | Cd | |
| 4-r | 45.86 | 38.06 | 0.253 | 0.019 | 0.139 | 0.089 | <0.004 | 0.616 | 0.032 | 14.84 | 0.005 | 2.05 | 0.381 | 35.7 | 0.959 | 66.6 | 0.613 | 11.4 | 31 | 6.46 | 69.8 | 3.21 | 43.2 | 17 | 0.186 | |
| 4-1 | 28.71 | 20.59 | 1.77 | 0.093 | 0.172 | 0.1 | <0.004 | 0.395 | 0.014 | 48.05 | 0.041 | 1.21 | 56.6 | 0.441 | 46.6 | 9.04 | 4.66 | 21.6 | 61.6 | 32.7 | 2.15 | 24.1 | 30.4 | 0.06 | ||
| 4-2 | 8.73 | 6.93 | 1.29 | 0.192 | 0.616 | 0.021 | 0.015 | 0.311 | 0.017 | 81.76 | 0.134 | 0.677 | 13 | 0.397 | 44.5 | 1.89 | 3.01 | 7.2 | 17.8 | 22.1 | 0.721 | 15 | 20.8 | 0.021 | ||
| 4-3 | 16.8 | 13.99 | 0.288 | 0.115 | 0.867 | 0.069 | 0.005 | 0.755 | 0.031 | 67.03 | 0.041 | 1.05 | 48.7 | 0.887 | 58.2 | 1.62 | 6.09 | 21.1 | 62 | 38.1 | 1.96 | 20.7 | 31.4 | 0.05 | ||
| 4-4g | 39.35 | 33.39 | 0.241 | 0.078 | 0.133 | 0.115 | <0.004 | 0.499 | 0.016 | 25.96 | 0.006 | 2.00 | 0.198 | 192 | 0.389 | 16.2 | 0.646 | 4.36 | 21.7 | 5.88 | 19.5 | 1.74 | 32.7 | 21.1 | 0.014 | |
| 4-5 | 10.26 | 8.55 | 0.435 | 0.115 | 0.723 | 0.026 | 0.007 | 0.367 | 0.077 | 79.37 | 0.068 | 3.13 | 39.2 | 0.481 | 35.4 | 1.93 | 4.17 | 16.9 | 11.6 | 28.3 | 1.37 | 16.2 | 179 | 0.021 | ||
| 4-6g | 27.37 | 22.96 | 0.315 | 0.096 | 0.209 | 0.075 | <0.004 | 0.677 | 0.051 | 47.82 | 0.012 | 2.02 | 0.576 | 115 | 0.907 | 23.7 | 1.03 | 4.98 | 25.7 | 8.45 | 16.2 | 2.86 | 27.6 | 26.4 | 0.016 | |
| 4-7 | 8.5 | 7.1 | 1.83 | 0.158 | 0.713 | 0.025 | 0.011 | 0.561 | 0.11 | 80.65 | 0.173 | 1.12 | 29.8 | 0.474 | 24.1 | 9.37 | 1.9 | 9.75 | 13.3 | 23.7 | 1.4 | 15.4 | 313 | 0.058 | ||
| 4-f | 40.11 | 34.86 | 0.487 | 0.046 | 0.207 | 0.081 | <0.004 | 0.906 | 0.03 | 23.25 | 0.010 | 1.95 | 0.235 | 237 | 0.353 | 24.2 | 0.652 | 4.42 | 25.6 | 8.07 | 30.8 | 1.64 | 30.2 | 23.7 | <0.002 | |
| Average | 14.60 | 11.43 | 1.123 | 0.135 | 0.618 | 0.048 | 0.0095 | 0.478 | 0.049 | 71.37 | 0.072 | 1.4374 | 37.46 | 0.536 | 41.76 | 4.77 | 3.966 | 15.31 | 33.26 | 28.98 | 1.5202 | 18.28 | 114.92 | 0.042 | ||
| 6-r | 52.42 | 26.69 | 1.82 | 0.149 | 0.118 | 0.462 | <0.004 | 1.22 | 0.035 | 16.96 | 0.026 | 3.33 | 0.27 | 101 | 0.156 | 21.8 | 2.08 | 4.36 | 24.6 | 16 | 65.9 | 1.81 | 34.7 | 42 | 0.078 | |
| 6-1 | 6.55 | 5.53 | 1.34 | 0.245 | 0.699 | 0.016 | 0.015 | 0.171 | 0.041 | 85.15 | 0.189 | 0.415 | 46.4 | 0.341 | 17.6 | 2.06 | 2.44 | 8.94 | 57.5 | 37.1 | 0.403 | 13.6 | 101 | 0.076 | ||
| 6-2 | 9.86 | 8.44 | 1.18 | 0.237 | 0.602 | 0.009 | 0.011 | 0.494 | 0.014 | 79.14 | 0.110 | 0.458 | 72.2 | 0.695 | 36.2 | 2.51 | 4.41 | 19 | 68.6 | 52.2 | 1.85 | 9.43 | 29.7 | 0.056 | ||
| 6-3g | 28.1 | 22.22 | 0.597 | 0.099 | 0.139 | 0.077 | 0.011 | 0.515 | 0.018 | 48.18 | 0.017 | 2.15 | 0.622 | 65 | 0.475 | 32.7 | 1.57 | 4.56 | 20.4 | 64.1 | 44.4 | 1.89 | 22.3 | 32.1 | 0.06 | |
| 6-4 | 5.54 | 4.58 | 1.56 | 0.667 | 2.41 | 0.006 | 0.025 | 0.117 | 0.013 | 84.79 | 0.458 | 0.268 | 40.1 | 0.192 | 15.1 | 2.85 | 2.01 | 5.07 | 61.3 | 112 | 0.687 | 7.29 | 26.4 | 0.087 | ||
| 6-5 | 6.84 | 5.7 | 0.779 | 0.365 | 0.824 | 0.009 | 0.012 | 0.11 | 0.01 | 85.06 | 0.157 | 0.326 | 34.3 | 0.18 | 20.8 | 2.8 | 4.54 | 14.9 | 51.1 | 36.9 | 0.496 | 10.2 | 26.7 | 0.054 | ||
| 6-6g | 28.14 | 22.33 | 0.15 | 0.087 | 0.114 | 0.081 | <0.004 | 0.53 | 0.02 | 48.52 | 0.007 | 2.14 | 0.481 | 65 | 0.465 | 34.4 | 1.43 | 4.87 | 22.2 | 8.78 | 19.2 | 2.05 | 22.6 | 27.8 | 0.021 | |
| 6-7 | 14.12 | 12.07 | 0.758 | 0.205 | 0.486 | 0.036 | 0.01 | 0.544 | 0.101 | 71.63 | 0.055 | 0.342 | 71.8 | 0.481 | 18.3 | 2.71 | 3.1 | 11.5 | 54.3 | 33.2 | 1.71 | 15.2 | 483 | 0.046 | ||
| 6-8 | 6.14 | 5.23 | 0.712 | 0.131 | 0.636 | 0.006 | 0.005 | 0.179 | 0.017 | 86.93 | 0.130 | 0.526 | 50.9 | 0.405 | 25.4 | 1.9 | 2.74 | 8.15 | 65.4 | 33 | 0.513 | 8.49 | 26.1 | 0.036 | ||
| 6-9 | 6.86 | 6.14 | 2.46 | 0.224 | 1.76 | 0.011 | 0.041 | 0.226 | 0.208 | 82 | 0.342 | 4.18 | 56.1 | 0.225 | 17.6 | 2.36 | 1.24 | 4.44 | 56.1 | 41.4 | 0.578 | 15.4 | 1111 | 0.093 | ||
| 6-10 | 10.31 | 8.8 | 0.36 | 0.116 | 0.251 | 0.022 | 0.008 | 0.472 | 0.022 | 79.5 | 0.038 | 0.389 | 77.5 | 0.842 | 32.6 | 1.77 | 4.2 | 18.5 | 63 | 32.9 | 1.6 | 9.59 | 30.7 | 0.043 | ||
| 6-11g | 40.36 | 34.83 | 0.156 | 0.03 | 0.132 | 0.096 | <0.004 | 1.21 | 0.018 | 22.91 | 0.004 | 1.97 | 0.357 | 196 | 1.06 | 18 | 3.41 | 6.09 | 16.2 | 15 | 21.2 | 7.09 | 30.1 | 43.9 | 0.004 | |
| 6-12 | 5.01 | 4.27 | 0.897 | 0.193 | 0.87 | 0.006 | 0.012 | 0.129 | 0.013 | 88.16 | 0.211 | 1.08 | 38.3 | 0.254 | 14.9 | 2.63 | 1.08 | 3.6 | 55.4 | 36.2 | 0.562 | 9.51 | 28.7 | 0.06 | ||
| 6-13 | 2.56 | 2.06 | 2.68 | 0.583 | 2.66 | 0.009 | 0.018 | 0.069 | 0.011 | 89.21 | 1.282 | 0.387 | 13.5 | 0.131 | 12.7 | 1.87 | 1.44 | 1.85 | 12.6 | 27.8 | 0.304 | 8.3 | 23.8 | 0.058 | ||
| 6-f | 52.8 | 26.59 | 0.592 | 0.164 | 0.123 | 0.443 | 0.01 | 1.06 | 0.031 | 18.14 | 0.011 | 3.37 | 0.87 | 109 | 0.169 | 17.8 | 1.49 | 2.68 | 20.3 | 57.7 | 75.3 | 1.74 | 32.3 | 46.8 | 0.146 | |
| Average | 7.38 | 6.28 | 1.273 | 0.297 | 1.120 | 0.013 | 0.0157 | 0.251 | 0.045 | 83.16 | 0.197 | 0.837 | 50.11 | 0.375 | 21.12 | 2.346 | 2.72 | 9.595 | 54.53 | 44.27 | 0.87 | 10.7 | 188.7 | 0.061 | ||
| World [31,32] | 8.47 | 5.98 | 4.85 | 0.22 | 1.23 | 0.19 | 0.015 | 0.33 | 0.092 | nd | nd | 1 | 14 | 1.1 | 9 | 2.1 | 1.9 | 3.2 | 16 | 28 | 0.99 | 6 | 100 | 0.2 | ||
| Sample | Be | As | Tl | Sc | Ni | V | Co | Ba | Cr | Cs | Rb | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu |
| 4-r | 1.36 | 0.216 | 0.054 | 11.3 | 2.51 | 15 | 2.23 | 27.7 | 1.63 | 0.247 | 3.58 | 86.7 | 155 | 16.8 | 57.4 | 10.1 | 1.61 | 8.37 | 1.55 | 8.32 | 46.6 | 1.56 | 4.79 | 0.78 | 4.88 | 0.72 |
| 4-1 | 4.56 | 5.22 | 4.54 | 8.47 | 8.36 | 20.3 | 3.48 | 36.7 | 9.95 | 1.22 | 5.5 | 40.9 | 73 | 8.22 | 29.1 | 5.1 | 0.9 | 4.51 | 0.85 | 4.56 | 23.4 | 0.87 | 2.57 | 0.43 | 2.79 | 0.43 |
| 4-2 | 1.87 | 0.348 | 0.325 | 5.15 | 9.72 | 12.3 | 5.75 | 21.5 | 8.6 | 0.118 | 0.946 | 38.6 | 64.5 | 7.13 | 25.4 | 4.53 | 0.8 | 3.75 | 0.63 | 3.3 | 16.3 | 0.61 | 1.86 | 0.29 | 1.79 | 0.26 |
| 4-3 | 2.47 | 0.411 | 0.196 | 11 | 9.05 | 27.3 | 2.76 | 32.6 | 9.69 | 0.661 | 3.28 | 34.8 | 62.4 | 7.18 | 25.7 | 5.24 | 0.81 | 5.01 | 1.09 | 6.43 | 30.4 | 1.23 | 3.71 | 0.59 | 3.78 | 0.51 |
| 4-4g | 1.72 | 0.224 | 0.042 | 10.3 | 5.74 | 22.3 | 1.19 | 27.4 | 9.39 | 0.826 | 5.45 | 21.6 | 36.9 | 4.24 | 14.8 | 3.08 | 0.58 | 3.56 | 0.88 | 5.27 | 26.1 | 1.03 | 3.07 | 0.51 | 3.27 | 0.46 |
| 4-5 | 2.78 | 0.445 | 0.271 | 6.07 | 10.2 | 13.4 | 6.41 | 61.1 | 5.43 | 0.208 | 1.42 | 52.4 | 72.2 | 7.41 | 25.4 | 4.16 | 0.59 | 3.67 | 0.74 | 4.32 | 21.5 | 0.83 | 2.56 | 0.39 | 2.42 | 0.35 |
| 4-6g | 3.05 | 0.259 | 0.158 | 10.3 | 6.23 | 21.2 | 1.42 | 33.2 | 8.93 | 0.753 | 3.98 | 35.3 | 72.9 | 8.86 | 32.4 | 6.34 | 0.93 | 5.31 | 1.04 | 5.49 | 24.8 | 1.02 | 2.9 | 0.47 | 3.03 | 0.41 |
| 4-7 | 4.28 | 1.33 | 0.787 | 3.89 | 12.6 | 14.5 | 6.71 | 70 | 6.2 | 0.147 | 0.929 | 49.4 | 73 | 8.26 | 29.9 | 5.11 | 0.73 | 3.71 | 0.69 | 3.8 | 18 | 0.74 | 2.32 | 0.37 | 2.45 | 0.33 |
| 4-f | 2.82 | 0.475 | 0.211 | 14.4 | 7.91 | 19.2 | 1.57 | 35.3 | 5.09 | 0.795 | 3.83 | 9.91 | 23.8 | 3.08 | 11.9 | 3.14 | 0.78 | 4.32 | 1.13 | 6.07 | 35.8 | 1.23 | 3.61 | 0.57 | 3.42 | 0.49 |
| Average | 3.192 | 1.5508 | 1.2238 | 6.916 | 9.986 | 17.56 | 5.022 | 44.38 | 7.974 | 0.4708 | 2.415 | 43.22 | 69.02 | 7.64 | 27.1 | 4.828 | 0.766 | 4.13 | 0.8 | 4.482 | 21.92 | 0.856 | 2.604 | 0.414 | 2.646 | 0.376 |
| 6-r | 1.29 | 1.32 | 0.787 | 9.85 | 18.1 | 65.9 | 4.72 | 87.5 | 91.9 | 3.28 | 20.3 | 66.2 | 114 | 14.3 | 51.9 | 8.01 | 0.78 | 5.7 | 0.84 | 3.62 | 16.4 | 0.63 | 1.84 | 0.29 | 1.88 | 0.29 |
| 6-1 | 1.14 | 1.736 | 0.199 | 3.68 | 4.08 | 12 | 1.33 | 25.5 | 4.65 | 0.074 | 0.786 | 28.3 | 45.4 | 4.49 | 13.8 | 2.05 | 0.34 | 1.85 | 0.34 | 1.87 | 8.56 | 0.34 | 1.01 | 0.15 | 0.93 | 0.13 |
| 6-2 | 1.24 | 0.799 | 0.132 | 5.7 | 7.57 | 19.5 | 1.08 | 21.2 | 7.38 | 0.044 | 0.673 | 17.5 | 29.6 | 3.21 | 11.4 | 2.43 | 0.4 | 2.19 | 0.46 | 2.66 | 12 | 0.5 | 1.48 | 0.23 | 1.46 | 0.21 |
| 6-3g | 1.65 | 0.489 | 0.091 | 7.19 | 13.9 | 25.8 | 2.42 | 3233 | 10.6 | 0.575 | 3.92 | 28 | 49 | 5.22 | 18.5 | 3.05 | 0.51 | 2.3 | 0.52 | 2.99 | 15.3 | 0.57 | 1.64 | 0.26 | 1.68 | 0.24 |
| 6-4 | 1.34 | 0.399 | 0.059 | 2.12 | 20.3 | 8.29 | 3.42 | 15.5 | 3.8 | 0.038 | 0.66 | 12.4 | 21.1 | 2.28 | 8.53 | 1.68 | 0.32 | 1.56 | 0.32 | 1.62 | 7.94 | 0.32 | 0.91 | 0.15 | 0.92 | 0.13 |
| 6-5 | 0.997 | 0.816 | 0.127 | 2.76 | 15.7 | 9.75 | 2.76 | 18.7 | 3.5 | 0.051 | 0.418 | 7.02 | 11.7 | 1.31 | 4.71 | 1.06 | 0.2 | 1.04 | 0.23 | 1.37 | 6.88 | 0.28 | 0.82 | 0.14 | 0.88 | 0.12 |
| 6-6g | 1.7 | 0.296 | 0.049 | 7.45 | 12 | 24.8 | 2.26 | 92.3 | 10.4 | 0.59 | 3.98 | 24.9 | 38.4 | 4.02 | 12.9 | 2.12 | 0.42 | 2.27 | 0.53 | 3.02 | 14.1 | 0.58 | 1.71 | 0.28 | 1.74 | 0.24 |
| 6-7 | 0.935 | 0.5 | 0.086 | 4.48 | 5.54 | 22.1 | 1.51 | 49.7 | 8.16 | 0.204 | 1.46 | 29.7 | 49.4 | 5.38 | 19.2 | 3.06 | 0.45 | 1.95 | 0.34 | 1.89 | 8.92 | 0.36 | 1.1 | 0.18 | 1.24 | 0.16 |
| 6-8 | 1.04 | 0.555 | 0.247 | 3.42 | 8.08 | 13.2 | 1.19 | 16.9 | 4.18 | 0.032 | 0.326 | 8.32 | 16.1 | 1.75 | 5.83 | 1.27 | 0.21 | 1.33 | 0.29 | 1.65 | 7.8 | 0.32 | 0.97 | 0.16 | 1 | 0.14 |
| 6-9 | 0.662 | 0.724 | 0.243 | 2.79 | 3.5 | 11.2 | 1.8 | 118 | 3.56 | 0.101 | 1.07 | 29.1 | 48 | 5.49 | 19.8 | 3.45 | 0.58 | 2.52 | 0.4 | 1.9 | 8.97 | 0.35 | 0.98 | 0.15 | 1.01 | 0.14 |
| 6-10 | 1.28 | 0.305 | 0.059 | 5.1 | 3.32 | 19.7 | 0.529 | 29.9 | 6.62 | 0.089 | 0.831 | 10.7 | 20.2 | 2.39 | 9.12 | 1.98 | 0.32 | 1.9 | 0.42 | 2.52 | 12 | 0.48 | 1.44 | 0.23 | 1.47 | 0.21 |
| 6-11g | 0.639 | 0.195 | 0.071 | 6.24 | 1.57 | 30.2 | 0.42 | 102 | 5.22 | 0.994 | 5.74 | 22.7 | 31.6 | 2.6 | 6.46 | 0.89 | 0.15 | 1.01 | 0.24 | 1.27 | 6.61 | 0.26 | 0.8 | 0.13 | 0.83 | 0.12 |
| 6-12 | 0.782 | 0.356 | 0.096 | 1.94 | 6.46 | 10.8 | 1.27 | 19.8 | 3.19 | 0.053 | 0.399 | 9.83 | 18.3 | 1.84 | 6.62 | 1.32 | 0.21 | 1.16 | 0.25 | 1.33 | 5.88 | 0.24 | 0.71 | 0.12 | 0.76 | 0.11 |
| 6-13 | 1.39 | 1.57 | 0.25 | 2.32 | 17.1 | 6.77 | 5.55 | 15.8 | 4.75 | 0.067 | 0.581 | 12.7 | 21.9 | 2.41 | 8.69 | 1.81 | 0.32 | 1.68 | 0.31 | 1.69 | 8.36 | 0.3 | 0.87 | 0.14 | 0.84 | 0.11 |
| 6-f | 1.48 | 0.259 | 0.136 | 9.54 | 20.1 | 64.5 | 4.29 | 69.28 | 85.2 | 3.39 | 21.4 | 55.4 | 90.6 | 11.4 | 40.6 | 6.29 | 0.42 | 4.77 | 0.76 | 3.68 | 19.3 | 0.67 | 2.15 | 0.33 | 2.21 | 0.34 |
| Average | 1.081 | 0.676 | 0.15 | 3.431 | 9.165 | 13.33 | 2.044 | 33.1 | 4.979 | 0.075 | 0.72 | 16.56 | 28.17 | 3.055 | 10.77 | 2.011 | 0.335 | 1.718 | 0.336 | 1.85 | 8.731 | 0.349 | 1.029 | 0.165 | 1.051 | 0.146 |
| World [31,32] | 2 | 8.3 | 0.58 | 3.7 | 17 | 28 | 6 | 150 | 17 | 1.1 | 18 | 11 | 23 | 3.4 | 12 | 2.2 | 0.43 | 2.7 | 0.31 | 2.1 | 8.4 | 0.57 | 1 | 0.3 | 1 | 0.2 |
| Coal | Elements | Vtrinite | Inertinite | Liptinite | Ad | Fe2O3 | U | Th | Cu | Ga | SiO2 | Al2O3 | CaO | K2O | TiO2 | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 4 | Cu | −0.98 | 0.97 | 0.64 | 0.87 | −0.10 | 0.79 | 0.80 | 1.00 | 0.93 | 0.86 | 0.90 | −0.35 | 0.94 | 0.47 | 0.79 |
| Pb | −0.66 | 0.63 | 0.65 | 0.50 | −0.49 | 0.87 | 0.57 | 0.79 | 0.61 | 0.51 | 0.56 | −0.03 | 0.60 | 0.32 | 1.00 | |
| No. 6 | Cu | −0.49 | 0.44 | 0.40 | 0.31 | −0.63 | 0.40 | 0.52 | 1.00 | 0.10 | 0.50 | 0.51 | −0.11 | 0.12 | 0.21 | 0.58 |
| Pb | −0.05 | −0.04 | 0.25 | 0.35 | −0.52 | 0.78 | 0.87 | 0.58 | −0.16 | 0.51 | 0.51 | 0.37 | −0.25 | −0.36 | 1.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhou, A.; Zeng, F.; Zhao, F.; Zou, Y. The Petrography, Mineralogy and Geochemistry of Some Cu- and Pb-Enriched Coals from Jungar Coalfield, Northwestern China. Minerals 2018, 8, 5. https://doi.org/10.3390/min8010005
Liu D, Zhou A, Zeng F, Zhao F, Zou Y. The Petrography, Mineralogy and Geochemistry of Some Cu- and Pb-Enriched Coals from Jungar Coalfield, Northwestern China. Minerals. 2018; 8(1):5. https://doi.org/10.3390/min8010005
Chicago/Turabian StyleLiu, Dongna, Anchao Zhou, Fangui Zeng, Fenghua Zhao, and Yu Zou. 2018. "The Petrography, Mineralogy and Geochemistry of Some Cu- and Pb-Enriched Coals from Jungar Coalfield, Northwestern China" Minerals 8, no. 1: 5. https://doi.org/10.3390/min8010005
APA StyleLiu, D., Zhou, A., Zeng, F., Zhao, F., & Zou, Y. (2018). The Petrography, Mineralogy and Geochemistry of Some Cu- and Pb-Enriched Coals from Jungar Coalfield, Northwestern China. Minerals, 8(1), 5. https://doi.org/10.3390/min8010005