Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
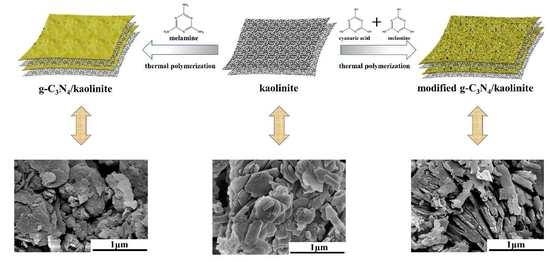
2.2. Catalysts Preparation
2.3. Characterization
2.4. Photoactivity Measurements
2.5. Computational Method
3. Results and Discussion
3.1. XRD Analyses
3.2. Morphology Analysis
3.3. BET Analysis
3.4. Optical Properties
3.5. FT-IR Analysis
3.6. DFT Analysis
3.7. Photocatalytic Performance
3.8. Possible Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chi, C.; Chen, W.; Guo, M.; Weng, M.; Yan, G.; Shen, X. Law and features of TVOC and Formaldehyde pollution in urban indoor air. Atmos. Environ. 2016, 132, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, P.; Zhang, Q.; Ma, H.; Hou, J.; Kong, X. Indoor Air Pollution and Human Perception in Public Buildings in Tianjin, China. Procedia Eng. 2015, 121, 552–557. [Google Scholar] [CrossRef]
- Luengas, A.; Barona, A.; Hort, C.; Gallastegui, G.; Platel, V.; Elias, A. A review of indoor air treatment technologies. Rev. Environ. Sci. Bio/Technol. 2015, 14, 499–522. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Zhao, H.; Yang, H. Microbial community and N removal of aerobic granular sludge at high COD and N loading rates. Bioresour. Technol. 2013, 143, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO Solid Solution as a Photocatalyst for Visible-Light-Driven Overall Water Splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Jayamohan, H.; Smith, Y.R.; Hansen, L.C.; Mohanty, S.K.; Gale, B.K.; Misra, M. Anodized titania nanotube array microfluidic device for photocatalytic application: Experiment and simulation. Appl. Catal. B Environ. 2015, 174–175, 167–175. [Google Scholar] [CrossRef]
- Dong, H.; Guo, X.; Yang, C.; Ouyang, Z. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl. Catal. B Environ. 2018, 230, 65–76. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Fang, L.; Ohfuji, H.; Shinmei, T.; Irifune, T. Experimental study on the stability of graphitic C3N4 under high pressure and high temperature. Diam. Relat. Mater. 2011, 20, 819–825. [Google Scholar] [CrossRef]
- Wang, M.; Cui, S.; Yang, X.; Bi, W. Synthesis of g-C3N4/Fe3O4 nanocomposites and application as a new sorbent for solid phase extraction of polycyclic aromatic hydrocarbons in water samples. Talanta 2015, 132, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Kundoo, S.; Banerjee, A.N.; Saha, P.; Chattopadhyay, K.K. Synthesis of crystalline carbon nitride thin films by electrolysis of methanol–urea solution. Mater. Lett. 2003, 57, 2193–2197. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Zhang, Y.; Fang, J.; Zhou, Y.; Yuan, S.; Zhang, C.; Chen, W. One-pot synthesis of K-doped g-C3N4 nanosheets with enhanced photocatalytic hydrogen production under visible-light irradiation. Appl. Surf. Sci. 2018, 440, 258–265. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.; Park, T.J. Novel visible light active graphitic C3N4–TiO2 composite photocatalyst: Synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B Environ. 2013, 142–143, 718–728. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving Carbon Nitride Photocatalysis by Supramolecular Preorganization of Monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J. Hydrothermal Synthesis of Graphene-TiO2 Nanotube Composites with Enhanced Photocatalytic Activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Leng, X.; Sun, Z.; Zheng, S. Characterization and improved solar light activity of vanadium doped TiO2/diatomite hybrid catalysts. J. Hazard. Mater. 2015, 285, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Y.; Zheng, S.; Park, Y.; Frost, R.L. Preparation and thermal energy storage properties of paraffin/calcined diatomite composites as form-stable phase change materials. Thermochim. Acta 2013, 558, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Sun, Z.; Duan, Y.; Ma, R.; Zheng, S. Synthesis of nano-TiO2/diatomite composite and its photocatalytic degradation of gaseous formaldehyde. Appl. Surf. Sci. 2017, 412, 105–112. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Huang, W.; Zheng, S. Facile synthesis of g-C3N4/montmorillonite composite with enhanced visible light photodegradation of rhodamine B and tetracycline. J. Taiwan Inst. Chem. Eng. 2016, 66, 363–371. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, G.; Zhang, X.; Zheng, S.; Frost, R.L. Enhanced visible-light photocatalytic activity of kaolinite/g-C3N4 composite synthesized via mechanochemical treatment. Appl. Clay Sci. 2016, 129, 7–14. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2 /kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Zhang, X.; Li, C.; Zheng, S. Construction of BiOCl/g-C3N4 /kaolinite composite and its enhanced photocatalysis performance under visible-light irradiation. J. Taiwan Inst. Chem. Eng. 2018, 84, 203–211. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Li, X.; Liu, L.; Zheng, S. Facile fabrication of g-C3N4/precipitated silica composite with enhanced visible-light photoactivity for the degradation of rhodamine B and Congo red. Adv. Power Technol. 2016, 27, 2051–2060. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Li, C.; Du, X.; Zheng, S.; Wang, G. Facile synthesis of two clay minerals supported graphitic carbon nitride composites as highly efficient visible-light-driven photocatalysts. J. Colloid Interface Sci. 2018, 511, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Durkin, D.P.; Elenewski, J.E.; Sun, Y.; Banek, N.A.; Hua, L.; Chen, H.; Wagner, M.J.; Zhang, W.; Shuai, D. Visible-light-responsive graphitic carbon nitride: Rational design and photocatalytic applications for water treatment. Environ. Sci. Technol. 2016, 50, 12938–12948. [Google Scholar] [CrossRef] [PubMed]










| Samples | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| KA | 22.957 | 0.059 | 6.979 |
| CN | 29.672 | 0.066 | 7.100 |
| CN/KA | 27.378 | 0.062 | 6.610 |
| m-CN/KA-0.5 | 28.562 | 0.063 | 6.591 |
| m-CN/KA-1 | 33.835 | 0.066 | 6.445 |
| m-CN/KA-2 | 40.632 | 0.076 | 6.434 |
| m-CN/KA-3 | 44.519 | 0.082 | 6.309 |
| m-CN/KA-4 | 49.522 | 0.088 | 6.251 |
| Sample | k (min−1) | Correlation Coefficient (R2) |
|---|---|---|
| P25 | 0.0012 | 0.99 |
| CN | 0.0021 | 0.99 |
| KA | 2.502 × 10−4 | 0.87 |
| CN/KA | 0.0043 | 0.99 |
| m-CN/KA-0.5 | 0.0048 | 0.99 |
| m-CN/KA-1 | 0.0059 | 0.99 |
| m-CN/KA-2 | 0.0064 | 0.97 |
| m-CN/KA-3 | 0.0074 | 0.98 |
| m-CN/KA-4 | 0.0086 | 0.98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Yuan, F.; Li, X.; Li, C.; Xu, J.; Wang, B. Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity. Minerals 2018, 8, 437. https://doi.org/10.3390/min8100437
Sun Z, Yuan F, Li X, Li C, Xu J, Wang B. Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity. Minerals. 2018; 8(10):437. https://doi.org/10.3390/min8100437
Chicago/Turabian StyleSun, Zhiming, Fang Yuan, Xue Li, Chunquan Li, Jie Xu, and Bin Wang. 2018. "Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity" Minerals 8, no. 10: 437. https://doi.org/10.3390/min8100437
APA StyleSun, Z., Yuan, F., Li, X., Li, C., Xu, J., & Wang, B. (2018). Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity. Minerals, 8(10), 437. https://doi.org/10.3390/min8100437