Application of Depletion Attraction in Mineral Flotation: II. Effects of Depletant Concentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Characterization of the Samples
Surface Tension of PEG
2.3. Flotation
2.3.1. Depletion Effects (Single Minerals of Silica and Malachite, Respectively and a Mixture of the Two)
2.3.2. Added Collector with PEG in a Binary System
2.4. Adsorption Experiments for PEG on Minerals
2.5. Viscosity Measurement of PEG Solution
3. Results and Discussion
3.1. Flotation Results
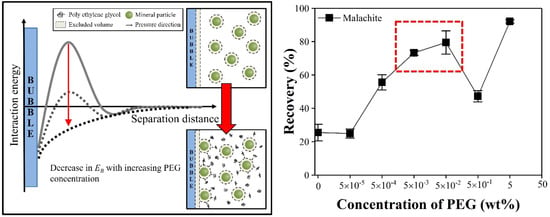
3.1.1. Single Minerals of Silica and Malachite
3.1.2. Mixture of Malachite and Silica
3.2. Flotation with a Small Amount of Collector with PEG in a Binary System
3.3. Viscosity Effect of a High PEG Concentration and Polyelectrolytes as a Depletant
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.; Kim, G.; Choi, S.; Kim, K.; Han, Y.; Bradford, S.A.; Choi, S.Q.; Kim, H. Application of depletion attraction in mineral flotation: I. Theory. Minerals 2018, in press. [Google Scholar]
- Mehrabani, J.V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Ghorbani, A. Process optimization and modelling of sphalerite flotation from a low-grade Zn-Pb ore using response surface methodology. Sep. Purif. Technol. 2010, 72, 242–249. [Google Scholar] [CrossRef]
- Thella, J.S.; Mukherjee, A.K.; Srikakulapu, N.G. Processing of high alumina iron ore slimes using classification and flotation. Powder Technol. 2012, 217, 418–426. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Fornasiero, D.; Filippov, L. Upgrading nickel in laterite ores by flotation. Miner. Eng. 2018, 121, 100–106. [Google Scholar] [CrossRef]
- Kim, G.; Choi, J.; Silva, R.A.; Song, Y.; Kim, H. Feasibility of bench-scale selective bioflotation of copper oxide minerals using rhodococcus opacus. Hydrometallurgy 2017, 168, 94–102. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.; Chae, W.; Kim, S.B.; Kim, H. Electrostatically controlled enrichment of lepidolite via flotation. Mater. Trans. 2012, 53, 2191–2194. [Google Scholar] [CrossRef]
- Lee, K.; Archibald, D.; McLean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Choi, J.; Park, K.; Hong, J.; Park, J.; Kim, H. Arsenic removal from mine tailings for recycling via flotation. Mater. Trans. 2013, 54, 2291–2296. [Google Scholar] [CrossRef]
- Choi, J.; Lee, E.; Choi, S.Q.; Lee, S.; Han, Y.; Kim, H. Arsenic removal from contaminated soils for recycling via oil agglomerate flotation. Chem. Eng. J. 2016, 285, 207–217. [Google Scholar] [CrossRef]
- Park, K.; Choi, J.; Gomez-Flores, A.; Kim, H. Flotation behavior of arsenopyrite and pyrite, and their selective separation. Mater. Trans. 2015, 56, 435–440. [Google Scholar] [CrossRef]
- Dopson, M.; Sundkvist, J.E.; Lindstrom, B. Toxicity of metal extraction and flotation chemicals to sulfolobus metallicus and chalcopyrite bioleaching. Hydrometallurgy 2006, 81, 205–213. [Google Scholar] [CrossRef]
- Tuovinen, O.H. Inhibition of Thiobacillus ferrooxidans by mineral flotation reagents. Eur. J. Appl. Microbiol. Biotechnol. 1978, 5, 301–304. [Google Scholar] [CrossRef]
- Webb, M.; Ruber, H.; Leduc, G. The toxicity of various mining flotation reagents to rainbow trout (Salmo gairdneri). Water Res. 1976, 10, 303–306. [Google Scholar] [CrossRef]
- Botero, A.E.C.; Torem, M.L.; de Mesquita, L.M.S. Fundamental studies of rhodococcus opacus as a biocollector of calcite and magnesite. Miner. Eng. 2007, 20, 1026–1032. [Google Scholar] [CrossRef]
- Grano, S.R.; Sollaart, M.; Skinner, W.; Prestidge, C.A.; Ralston, J. Surface modifications in the chalcopyrite-sulphite ion system. І. Collectorless flotation, XPS and dissolution study. Int. J. Miner. Process. 1997, 50, 1–26. [Google Scholar] [CrossRef]
- Merma, A.G.; Torem, M.L.; Morán, J.J.V.; Monte, M.B.M. On the fundamental aspects of apatite and quartz flotation using a gram positive strain as a bioreagent. Miner. Eng. 2013, 48, 61–67. [Google Scholar] [CrossRef]
- Nagaoka, T.; Ohmura, N.; Saiki, H. A novel mineral flotation process using thiobacillus ferrooxidans. Appl. Environ. Microbiol. 1999, 65, 3588–3593. [Google Scholar] [PubMed]
- Patra, P.; Natarajan, K.A. Microbially induced flocculation and flotation for separation of chalcopyrite from quartz and calcite. Int. J. Miner. Process. 2004, 74, 143–155. [Google Scholar] [CrossRef]
- Patra, P.; Natarajan, K.A. Microbially induced flotation and flocculation of pyrite and sphalerite. Colloid Surf. B Biointerfaces 2004, 36, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.A.; Ralston, J. The collectorless flotation and separation of sulphide minerals by Eh control. Int. J. Miner. Process. 1988, 23, 55–84. [Google Scholar] [CrossRef]
- Luttrell, G.H.; Yoon, R.-H. The collectorless flotation of chalcopyrite ores using sodium sulfide. Int. J. Miner. Process. 1984, 13, 271–283. [Google Scholar] [CrossRef]
- Luttrell, G.H.; Yoon, R.H. Surface studies of the collectorless flotation of chalcopyrite. Colloid Surf. 1984, 12, 239–254. [Google Scholar] [CrossRef]
- Hong, G.; Choi, J.; Han, Y.; Yoo, K.-S.; Kim, K.; Kim, S.B.; Kim, H. Relationship between surface characteristics and floatability in representative sulfide minerals: Role of surface oxidation. Mater. Trans. 2017, 58, 1069–1075. [Google Scholar] [CrossRef]
- Ekmekci, Z.; Demirel, H. Effects of galvanic interaction on collectorless flotation behaviour of chalcopyrite and pyrite. Int. J. Miner. Process. 1997, 52, 31–48. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Sabacky, B.J. On the natural floatability of sulfides. Int. J. Miner. Process. 1981, 8, 79–84. [Google Scholar] [CrossRef]
- Fielden, M.L.; Hayes, R.A.; Ralston, J. Surface and capillary forces affecting air bubble-particle interactions in aqueous electrolyte. Langmuir 1996, 12, 3721–3727. [Google Scholar] [CrossRef]
- Hewitt, D.; Fornasiero, D.; Ralston, J. Bubble particle attachment efficiency. Miner. Eng. 1994, 7, 657–665. [Google Scholar] [CrossRef]
- Nguyen, A.V. Hydrodynamics of liquid flows around air bubbles in flotation: A review. Int. J. Miner. Process. 1999, 56, 165–205. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Evans, G.M. Attachment interaction between air bubbles and particles in froth flotation. Exp. Therm. Fluid Sci. 2004, 28, 381–385. [Google Scholar] [CrossRef]
- Ralston, J.; Fornasiero, D.; Hayes, R. Bubble–particle attachment and detachment in flotation. Int. J. Miner. Process. 1999, 56, 133–164. [Google Scholar] [CrossRef]
- Schulze, H.J. Hydrodynamics of bubble-mineral particle collisions. Miner. Process Extr. Metall. Rev. 1989, 5, 43–76. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 1954, 22, 1255–1256. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. Interaction between particles suspended in solutions of macromolecules. J. Polym. Sci. 1958, 33, 183–192. [Google Scholar] [CrossRef]
- Mao, Y.; Cates, M.E.; Lekkerkerker, H.N.W. Depletion force in colloidal systems. Physica A 1995, 222, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, S.; Ryu, J.; Jeon, J.; Jang, S.G.; Kim, H.; Gweon, D.G.; Im, W.B.; Han, Y.; Kim, H.; et al. Processable high internal phase pickering emulsions using depletion attraction. Nat. Commun. 2017, 8, 14305. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, S.; Choi, J.; Kim, G.; Tong, M.P.; Kim, H. Influence of excess sulfide ions on the malachite-bubble interaction in the presence of thiol-collector. Sep. Purif. Technol. 2016, 168, 1–7. [Google Scholar] [CrossRef]
- Kim, G.; Park, K.; Choi, J.; Gomez-Flores, A.; Han, Y.; Choi, S.Q.; Kim, H. Bioflotation of malachite using different growth phases of rhodococcus opacus: Effect of bacterial shape on detachment by shear flow. Int. J. Miner. Process. 2015, 143, 98–104. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.Q.; Park, K.; Han, Y.; Kim, H. Flotation behaviour of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Kim, H.N.; Bradford, S.A.; Walker, S.L. Escherichia coli O157:H7 transport in saturated porous media: Role of solution chemistry and surface macromolecules. Environ. Sci. Technol. 2009, 43, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hwang, G.; Kim, D.; Bradford, S.A.; Lee, B.; Eom, I.; Kim, P.J.; Choi, S.Q.; Kim, H. Transport, retention, and long-term release behavior of ZnO nanoparticle aggregates in saturated quartz sand: Role of solution pH and biofilm coating. Water Res. 2016, 90, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Bartell, F.E.; Miller, F.L. A method for the measurement of interfacial tension of liquid-liquid systems. J. Am. Chem. Soc. 1928, 50, 1961–1967. [Google Scholar] [CrossRef]
- Somasundaran, P.; Zhang, L. Adsorption of surfactants on minerals for wettability control in improved oil recovery processes. J. Pet. Sci. Eng. 2006, 52, 198–212. [Google Scholar] [CrossRef]
- Hwang, G.; Gomez-Flores, A.; Bradford, S.A.; Choi, S.; Jo, E.; Kim, S.B.; Tong, M.P.; Kim, H. Analysis of stability behavior of carbon black nanoparticles in ecotoxicological media: Hydrophobic and steric effects. Colloid Surf. A Physicochem. Eng. Asp. 2018, 554, 306–316. [Google Scholar] [CrossRef]
- Herrmann, A.; Clague, M.J.; Blumenthal, R. Enhancement of viral fusion by nonadsorbing polymers. Biophys. J. 1993, 65, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Moudgil, B.M. Adsorption mechanism(s) of poly(ethylene oxide) on oxide surfaces. J. Colloid Interface Sci. 1997, 196, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.H.; Kim, M.W. Molecular-weight dependence of the surface-tension of aqueous poly(ethylene oxide) solutions. Faraday Discuss. 1994, 98, 245–252. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Lodge, T. Polymer Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 608. [Google Scholar]
- Drzymala, J.; Lekki, J.; Kielkowska, M.M. A study of the germanium—Sodium oleate flotation system. Powder Technol. 1987, 52, 251–256. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Avranas, A. Treatment of oil-in-water emulsions by coagulation and dissolved-air flotation. Colloid Surf. A Physicochem. Eng. Asp. 2000, 172, 153–161. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, W.; Deng, J.; Chen, B.; Yan, W.; Xiong, S.; Huang, Y.; Liu, J. Flotation behaviors of ilmenite, titanaugite, and forsterite using sodium oleate as the collector. Miner. Eng. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Cohen, J.A.; Podgornik, R.; Hansen, P.L.; Parsegian, V.A. A phenomenological one-parameter equation of state for osmotic pressures of PEG and other neutral flexible polymers in good solvents. J. Phys. Chem. B 2009, 113, 3709–3714. [Google Scholar] [CrossRef] [PubMed]






| Concentration of PEG (wt %) | Before Adsorption | After Adsorption onto Silica | After Adsorption onto Malachite |
|---|---|---|---|
| 5 × 10−2 | 0.093 ± 0.006 | 0.100 ± 0.012 | 0.101 ± 0.002 |
| 5 × 10−1 | 0.743 ± 0.011 | 0.759 ± 0.003 | 0.773 ± 0.010 |
| 5 | 1.417 ± 0.068 | 1.432 ± 0.028 | 1.558 ± 0.125 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Choi, J.; Choi, S.; Kim, K.; Han, Y.; Bradford, S.A.; Choi, S.Q.; Kim, H. Application of Depletion Attraction in Mineral Flotation: II. Effects of Depletant Concentration. Minerals 2018, 8, 450. https://doi.org/10.3390/min8100450
Kim G, Choi J, Choi S, Kim K, Han Y, Bradford SA, Choi SQ, Kim H. Application of Depletion Attraction in Mineral Flotation: II. Effects of Depletant Concentration. Minerals. 2018; 8(10):450. https://doi.org/10.3390/min8100450
Chicago/Turabian StyleKim, Gahee, Junhyun Choi, Sowon Choi, KyuHan Kim, Yosep Han, Scott A. Bradford, Siyoung Q. Choi, and Hyunjung Kim. 2018. "Application of Depletion Attraction in Mineral Flotation: II. Effects of Depletant Concentration" Minerals 8, no. 10: 450. https://doi.org/10.3390/min8100450
APA StyleKim, G., Choi, J., Choi, S., Kim, K., Han, Y., Bradford, S. A., Choi, S. Q., & Kim, H. (2018). Application of Depletion Attraction in Mineral Flotation: II. Effects of Depletant Concentration. Minerals, 8(10), 450. https://doi.org/10.3390/min8100450