The Role of ε-Fe2O3 Nano-Mineral and Domains in Enhancing Magnetic Coercivity: Implications for the Natural Remanent Magnetization
Abstract
:1. Introduction
2. Samples
3. Experimental Methods
3.1. Enrichment of Luogufengite
3.2. Techniques
4. Results and Discussion
4.1. Luogufengite Nano-Mineral with Giant Coercive Field
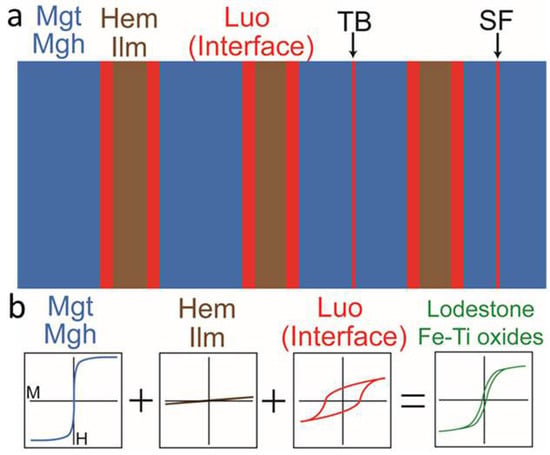
4.2. Luogufengite-Like Nano-Domains in Lodestone and Fe-Ti Oxides
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robinson, P.; McEnroe, S.A.; Miyajima, N.; Fabian, K.; Church, N. Remanent magnetization, magnetic coupling and interface ionic configurations of intergrown rhombohedral and cubic Fe-Ti oxides: A short survey. Am. Mineral. 2016, 101, 518–530. [Google Scholar] [CrossRef]
- Robinson, P.; Harrison, R.J.; McEnroe, S.A.; Hargraves, R.B. Lamellar magnetism in the haematite-ilmenite series as an explanation for strong remanent magnetization. Nature 2002, 418, 517–520. [Google Scholar] [CrossRef] [PubMed]
- McCammon, C.A.; McEnroe, S.A.; Robinson, P.; Fabian, K.; Burton, B.P. High efficiency of natural lamellar remanent magnetisation in single grains of ilmeno-hematite calculated using mossbauer spectroscopy. Earth Planet. Sci. Lett. 2009, 288, 268–278. [Google Scholar] [CrossRef]
- McEnroe, S.A.; Fabian, K.; Robinson, P.; Gaina, C.; Brown, L.L. Crustal magnetism, lamellar magnetism and rocks that remember. Elements 2009, 5, 241–246. [Google Scholar] [CrossRef]
- Acuna, M.H.; Connerney, J.E.P.; Wasilewski, P.; Lin, R.P.; Anderson, K.A.; Carlson, C.W.; McFadden, J.; Curtis, D.W.; Mitchell, D.; Reme, H.; et al. Magnetic field and plasma observations at Mars: Initial results of the mars global surveyor mission. Science 1998, 279, 1676–1680. [Google Scholar] [PubMed]
- Connerney, J.E.P.; Acuna, M.H.; Ness, N.F.; Spohn, T.; Schubert, G. Mars crustal magnetism. Space Sci. Rev. 2004, 111, 1–32. [Google Scholar] [CrossRef]
- Fabian, K.; McEnroe, S.A.; Robinson, P.; Shcherbakov, V.P. Exchange bias identifies lamellar magnetism as the origin of the natural remanent magnetization in titanohematite with ilmenite exsolution from Modum, Norway. Earth Planet. Sci. Lett. 2008, 268, 339–353. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.; McIntosh, G.; Osete, M.L.; del Campo, A.; Villalain, J.J.; Perez, L.; Kovacheva, M.; de la Fuente, O.R. Epsilon iron oxide: Origin of the high coercivity stable low curie temperature magnetic phase found in heated archeological materials. Geochem. Geophys. Geosyst. 2017, 18, 2646–2656. [Google Scholar] [CrossRef]
- Fabian, K.; Shcherbakov, V.P.; McEnroe, S.A.; Robinson, P.; Burton, B.P. Magnetic mean-field modelling of solid solutions: Theoretical foundations and application to the hematite-ilmenite system. Geophys. J. Int. 2015, 202, 1029–1040. [Google Scholar] [CrossRef]
- Ramdohr, P. The Ore Minerals and Their Intergrowths, 2nd ed.; Pergamon Press: Oxford, UK, 1980; pp. 85–110. [Google Scholar]
- Lattard, D. Experimental-evidence for the exsolution of ilmenite from titaniferous spinel. Am. Mineral. 1995, 80, 968–981. [Google Scholar] [CrossRef]
- McEnroe, S.A.; Robinson, P.; Miyajima, N.; Fabian, K.; Dyar, D.; Sklute, E. Lamellar magnetism and exchange bias in billion-year-old titanohematite with nanoscale ilmenite exsolution lamellae: I. Mineral and magnetic characterization. Geophys. J. Int. 2016, 206, 470–486. [Google Scholar] [CrossRef]
- Xu, H.F.; Lee, S.; Xu, H. Luogufengite: A new nano-mineral of Fe2O3 polymorph with giant coercive field. Am. Mineral. 2017, 102, 711–719. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Souza-Neto, N.M.; Haskel, D.; Gich, M.; Frontera, C.; Roig, A.; Veenendaal, M.; Nogués, J. Nonzero orbital moment in high coercivity ε-Fe2O3 and low-temperature collapse of the magnetocrystalline anisotropy. Phys. Rev. B 2009, 79, 094404. [Google Scholar] [CrossRef]
- Creighton, D. Menan buttes, southeastern idaho. Centen. Field Guide 1987, 2, 109–111. [Google Scholar]
- Doelling, H.H.; Davis, F.D.; Brandt, C.J. The Geology of Kane County, Utah: Geology, Mineral Resources, Geologic Hazards; Utah Geological Survey: Salt Lake City, UT, USA, 1989. [Google Scholar]
- Frey, F.A.; Gerlach, D.C.; Hickey, R.L.; Lopez-Escobar, L.; Munizaga-Villavicencio, F. Petrogenesis of the Laguna del Maule volcanic complex, Chile (36 s). Contrib. Mineral. Petrol. 1984, 88, 133–149. [Google Scholar] [CrossRef]
- Macdonald, G.A.; Abbott, A.T.; Peterson, F.L. Volcanoes in the Sea: The Geology of Hawaii; University of Hawaii Press: Honolulu, HI, USA, 1983. [Google Scholar]
- Jin, J.; Ohkoshi, S.; Hashimoto, K. Giant coercive field of nanometer-sized iron oxide. Adv. Mater. 2004, 16, 48–51. [Google Scholar] [CrossRef]
- Xu, H.; Lee, S. Luogufengite, IMA 2016-005. CNMNC Newsletter. Mineral. Mag. 2016, 80, 691–695. [Google Scholar]
- Lee, S.; Xu, H.F. Size-dependent phase map and phase transformation kinetics for nanometric Iron(III) oxides (γ → ε → α). J. Phys. Chem. C 2016, 120, 13316–13322. [Google Scholar] [CrossRef]
- Tucek, J.; Zboril, R.; Namai, A.; Ohkoshi, S. ε-Fe2O3: An advanced nanomaterial exhibiting giant coercive field, millimeter-wave ferromagnetic resonance and magnetoelectric coupling. Chem. Mater. 2010, 22, 6483–6505. [Google Scholar] [CrossRef]
- Dejoie, C.; Sciau, P.; Li, W.D.; Noe, L.; Mehta, A.; Chen, K.; Luo, H.J.; Kunz, M.; Tamura, N.; Liu, Z. Learning from the past: Rare ε-Fe2O3 in the ancient black-glazed Jian (Tenmoku) wares. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lee, S.; Xu, H.; Jacobs, R.; Morgan, D. Valleyite, IMA 2017–2026. Mineral. Mag. 2017, 81, 1035. [Google Scholar]
- Yoshikiyo, M.; Yamada, K.; Namai, A.; Ohkoshi, S. Study of the electronic structure and magnetic properties of ε-Fe2O3 by first-principles calculation and molecular orbital calculations. J. Phys. Chem. C 2012, 116, 8688–8691. [Google Scholar] [CrossRef]
- Blake, R.L.; Hessevick, R.E. Refinement of hematite structure. Am. Mineral. 1966, 51, 123–129. [Google Scholar]
- Shmakov, A.N.; Kryukova, G.N.; Tsybulya, S.V.; Chuvilin, A.L.; Solovyeva, L.P. Vacancy ordering in gamma-Fe2O3–Synchrotron X-ray-powder diffraction and high-resolution microscopy studies. J. Appl. Crystallogr. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Fjellvag, H.; Gronvold, F.; Stolen, S.; Hauback, B. On the crystallographic and magnetic structures of nearly stoichiometric iron monoxide. J. Solid State Chem. 1996, 124, 52–57. [Google Scholar] [CrossRef]
- Wechsler, B.A.; Prewitt, C.T. Crystal structure of ilmenite (FeTiO3) at high temperature and high pressure. Am. Mineral. 1984, 69, 176–185. [Google Scholar]
- Finger, L.W.; Hazen, R.M.; Hofmeister, A.M. High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): Comparisons with silicate spinels. Phys. Chem. Miner. 1986, 13, 215–220. [Google Scholar] [CrossRef]
- Ozdemir, O.; Dunlop, D.J.; Moskowitz, B.M. Changes in remanence, coercivity and domain state at low temperature in magnetite. Earth Planet. Sci. Lett. 2002, 194, 343–358. [Google Scholar] [CrossRef]
- Namai, A.; Yoshikiyo, M.; Yamada, K.; Sakurai, S.; Goto, T.; Yoshida, T.; Miyazaki, T.; Nakajima, M.; Suemoto, T.; Tokoro, H.; et al. Hard magnetic ferrite with a gigantic coercivity and high frequency millimetre wave rotation. Nat. Commun. 2012, 3, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, S.; Shimoyama, J.I.; Hashimoto, K.; Ohkoshi, S.I. Large coercive field in magnetic-field oriented ε-Fe2O3 nanorods. Chem. Phys. Lett. 2008, 458, 333–336. [Google Scholar] [CrossRef]
- Wasilewski, P.; Kletetschka, G. Lodestone: Natures only permanent magnet—What it is and how it gets charged. Geophys. Res. Lett. 1999, 26, 2275–2278. [Google Scholar] [CrossRef]
- Banfield, J.F.; Wasilewski, P.J.; Veblen, D.R. Tem study of relationships between the microstructures and magnetic-properties of strongly magnetized magnetite and maghemite. Am. Mineral. 1994, 79, 654–667. [Google Scholar]
- Gich, M.; Gazquez, J.; Roig, A.; Crespi, A.; Fontcuberta, J.; Idrobo, J.C.; Pennycook, S.J.; Varela, M.; Skumryev, V.; Varela, M. Epitaxial stabilization of ε-Fe2O3 (00L) thin films on SrTiO3 (111). Appl. Phys. Lett. 2010, 96, 112508. [Google Scholar] [CrossRef] [Green Version]
- Kirk, T.L.; Hellwig, O.; Fullerton, E.E. Coercivity mechanisms in positive exchange-biased co films and Co./Pt multilayers. Phys. Rev. B 2002, 65, 224426. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, S.H.; Li, J.; Wang, Z.L.; Liu, J.P. Tailoring magnetic properties of core/shell nanoparticles. Appl. Phys. Lett. 2004, 85, 792–794. [Google Scholar] [CrossRef]
- Darling, S.; Bader, S. A materials chemistry perspective on nanomagnetism. J. Mater. Chem. 2005, 15, 4189–4195. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Liu, J.P.; Wang, Z.L.; Sun, S. Exchange-coupled nanocomposite magnets by nanoparticle self-assembly. Nature 2002, 420, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, D.J.; Özdemir, Ö. Rock Magnetism: Fundamentals and Frontiers, 3rd ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 234–259. [Google Scholar]
- Putnis, A. An Introduction to Mineral Sciences; Cambridge University Press: Cambridge, UK, 1992; pp. 442–444. [Google Scholar]
- Langel, R.; Phillips, J.; Horner, R. Initial scalar magnetic anomaly map from magsat. Geophys. Res. Lett. 1982, 9, 269–272. [Google Scholar] [CrossRef]
- Lillis, R.; Frey, H.; Manga, M. Rapid decrease in martian crustal magnetization in the Noachian era: Implications for the dynamo and climate of early mars. Geophys. Res. Lett. 2008, 35, 14203. [Google Scholar] [CrossRef]
- Dunlop, D.J.; Arkani-Hamed, J. Magnetic minerals in the martian crust. J. Geophys. Res. Planets 2005, 110. [Google Scholar] [CrossRef]
- Louzada, K.L.; Stewart, S.T.; Weiss, B.P.; Gattacceca, J.; Lillis, R.J.; Halekas, J.S. Impact demagnetization of the Martian crust: Current knowledge and future directions. Earth Planet. Sci. Lett. 2011, 305, 257–269. [Google Scholar] [CrossRef]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Xu, H. The Role of ε-Fe2O3 Nano-Mineral and Domains in Enhancing Magnetic Coercivity: Implications for the Natural Remanent Magnetization. Minerals 2018, 8, 97. https://doi.org/10.3390/min8030097
Lee S, Xu H. The Role of ε-Fe2O3 Nano-Mineral and Domains in Enhancing Magnetic Coercivity: Implications for the Natural Remanent Magnetization. Minerals. 2018; 8(3):97. https://doi.org/10.3390/min8030097
Chicago/Turabian StyleLee, Seungyeol, and Huifang Xu. 2018. "The Role of ε-Fe2O3 Nano-Mineral and Domains in Enhancing Magnetic Coercivity: Implications for the Natural Remanent Magnetization" Minerals 8, no. 3: 97. https://doi.org/10.3390/min8030097
APA StyleLee, S., & Xu, H. (2018). The Role of ε-Fe2O3 Nano-Mineral and Domains in Enhancing Magnetic Coercivity: Implications for the Natural Remanent Magnetization. Minerals, 8(3), 97. https://doi.org/10.3390/min8030097