Performance Analysis of Thiocarbonohydrazide as a Novel Selective Depressant for Chalcopyrite in Molybdenite-Chalcopyrite Separation
Abstract
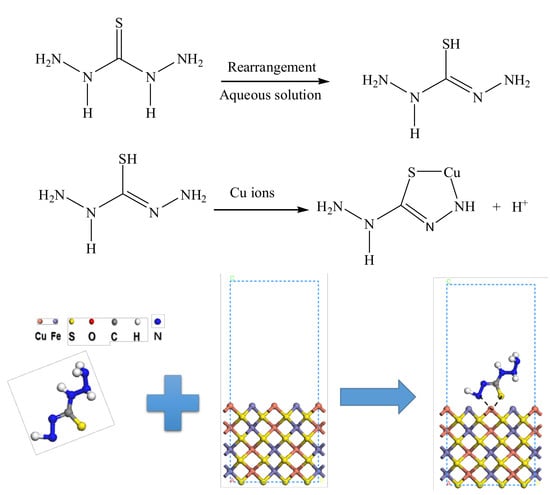
:1. Introduction
2. Materials and Methods
2.1. Reagents and Mineral Samples
2.2. Flotation Experiments
2.3. Zeta Potential Measurements
2.4. FT-IR Spectra Measurements
2.5. X-ray Photoelectron Spectroscopy Measurements
2.6. Time of Flight-Secondary Ion Mass Spectrometry
3. Results and Discussion
3.1. Flotation Tests
3.2. Zeta Potential Analysis
3.3. FTIR Spectra Measurements
3.4. XPS Analysis
3.5. ToF-SIMS Analysis
3.6. Adsorption Model
4. Conclusions
- (a)
- Flotation results indicated that TCH has a strong depressive strength and selectivity towards chalcopyrite and a negligible effect on the floatability of molybdenite at pH lower than 9.
- (b)
- Zeta potential measurements showed that the adsorption between TCH and chalcopyrite is chemisorption, and results into the formation of TCH–copper complexes. Further, after TCH treatment, the surface of chalcopyrite becomes more positively charged in the pH ranges from 4 to 8. The positive charges can be attributed to the protonated isomer structure of TCH, implying stronger interactions with copper ions.
- (c)
- FTIR analysis displayed the appearance of new vibrational bands on the chalcopyrite surface due to TCH–Cu2+ complexes, which were probably produced by the reaction of thiol and primary amine functional groups of TCH with copper atoms to form Cu–S, Cu–N bonds on the chalcopyrite surface.
- (d)
- Results of XPS and ToF-SIMS measurements further confirmed the chemisorption of TCH onto the chalcopyrite surface.
- (e)
- Based on this detailed investigation, it is suggested that thiocarbonohydrazide (TCH), due to its unique properties, such as double chelating groups, characteristic adsorption manner, good selectivity, environmental friendliness, etc., could be used as a good depressant for chalcopyrite in the flotation separation of molybdenum sulphide minerals.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Zhang, C. Separation of molybdenite from chalcopyrite in the presence of novel depressant 4-amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals 2017, 7, 146. [Google Scholar]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsorption studies. Miner. Eng. 2007, 20, 600–608. [Google Scholar] [CrossRef]
- Ming-Yang, L.I.; Wei, D.Z.; Shen, Y.B.; Liu, W.G.; Gao, S.L.; Liang, G.Q. Selective depression effect in flotation separation of copper-molybdenum sulfides using 2,3-disulfanylbutanedioic acid. Trans. Nonferrous Met. Soc. China 2015, 25, 3126–3132. [Google Scholar]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper-molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Wie, J.M.; Fuerstenau, D.W. The effect of dextrin on surface properties and the flotation of molybdenite. Int. J. Miner. Process. 1974, 1, 17–32. [Google Scholar] [CrossRef]
- Castro, S.; Laskowski, J.S. Depressing effect of flocculants on molybdenite flotation. Miner. Eng. 2015, 74, 13–19. [Google Scholar] [CrossRef]
- Lai, R.W.M.; Stone, L.C.; Rimmasch, B.E. Effect of humus organics on the flotation recovery of molybdenite. Int. J. Miner. Process. 1984, 12, 163–172. [Google Scholar] [CrossRef]
- Beaussart, A.; Parkinson, L.; Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of modified dextrins on molybdenite: Afm imaging, contact angle, and flotation studies. J. Colloid Interface Sci. 2012, 368, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Liu, R.; Chen, P.; Tian, M.J. Utilization of acetic acid-[(hydrazinylthioxomethyl)thio]-sodium as a novel selective depressant for chalcopyrite in the flotation separation of molybdenite. Sep. Purif. Technol. 2017, 179, 248–256. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Z.; Zheng, G.; Zhu, Y.; Wu, W. The design of a macromolecular depressant for galena based on dft studies and its application. Miner. Eng. 2017, 112, 50–56. [Google Scholar] [CrossRef]
- Chen, J.H.; Lan, L.H.; Liao, X.J. Depression effect of pseudo glycolythiourea acid in flotation separation of copper-molybdenum. Trans. Nonferrous Met. Soc. China 2013, 23, 824–831. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhai, J.; Guan, Q. Evaluation of the replacement of nacn with depressant mixtures in the separation of copper-molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Yin, Z.G.; Sun, W.; Hu, Y.; Guan, Q.J.; Zhang, C.H.; Gao, Y.S.; Zhai, J.H. Depressing behaviors and mechanism of disodium bis (carboxymethyl) trithiocarbonate on separation of chalcopyrite and molybdenite. Trans. Nonferrous Met. Soc. China 2017, 27, 883–890. [Google Scholar] [CrossRef]
- Piao, Z.J.; Wei, D.Z.; Liu, Z.L. Effects of small molecule organic depressants on the flotation behavior of chalcopyrite and galena. J. Northeast. Univ. 2013, 34, 884–888. [Google Scholar]
- Piao, Z.J.; Wei, D.Z.; Liu, Z.L. Influence of sodium 2,3-dihydroxypropyl dithiocarbonate on floatability of chalcopyrite and galena. Trans. Nonferrous Met. Soc. China 2014, 24, 3343–3347. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Liu, R.; Jiang, W.; Zhang, C.; Guan, Q.; Zhang, C. Synthesis of acetic acid-[(hydrazinylthioxome thyl) thio]-sodium and its application on the flotation separation of molybdenite from galena. J. Ind. Eng. Chem. 2017, 52, 82–88. [Google Scholar] [CrossRef]
- Du, S.; Luo, Z. Flotation technology of refractory low-grade molybdenum ore. Int. J. Min. Sci. Technol. 2013, 23, 255–260. [Google Scholar] [CrossRef]
- Singh, R.N.; Rawat, P.; Sahu, S. Vibrational spectra, electronic absorption, nonlinear optical properties, evaluation of bonding, chemical reactivity and thermodynamic properties of ethyl 4-(1-(2-(hydrazinecarbonothioyl)hydrazono)ethyl)-3,5-dimethyl-1h-pyrrole-2-carboxylate molecule by ab. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Abouhussein, A.A.; Linert, W. Synthesis, spectroscopic and biological activities studies of acyclic and macrocyclic mono and binuclear metal complexes containing a hard-soft schiff base. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Raparia, S.; Surain, P. Co(II), Ni(II), Cu(II) and Zn(II) complexes of 4-(4-cyanobenzylideneamino)-3-mercapto-5-oxo-1,2,4-triazine: Synthesis, characterization and biological studies. Med. Chem. Res. 2015, 24, 2336–2346. [Google Scholar] [CrossRef]
- Netalkar, P.P.; Netalkar, S.P.; Revankar, V.K. Transition metal complexes of thiosemicarbazone: Synthesis, structures and invitro antimicrobial studies. Polyhedron 2015, 100, 215–222. [Google Scholar] [CrossRef]
- Sen, A.K.; Singh, R.N.; Handa, R.N.; Dubey, S.N.; Squattrito, P.J. A structural comparison of some amine- and thione-substituted triazoles. J. Mol. Struct. 1998, 470, 61–69. [Google Scholar] [CrossRef]
- Rathi, P.; Singh, D.P. Template engineered biopotent macrocyclic complexes involving furan moiety: Molecular modeling and molecular docking. J. Mol. Struct. 2015, 1093, 201–207. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Puri, P.; Kumar, M.; Sharma, C. Cobalt, nickel, copper and zinc complexes with 1,3-diphenyl-1h-pyrazole-4-carboxaldehyde schiff bases: Antimicrobial, spectroscopic, thermal and fluorescence studies. Eur. J. Med. Chem. 2012, 52, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kajdan, T.W.; Squattrito, P.J.; Dubey, S.N. Coordination geometries of bis(4-amino-3-ethyl-1,2,4-triazole-5-thione) complexes of Mn, Fe, Co, Ni, Cu and Zn: Relationship to the 3-methyl analogs. Inorg. Chim. Acta 2000, 300, 1082–1089. [Google Scholar] [CrossRef]
- Menzies, C.M.; Squattrito, P.J. Coordination geometries of bis(4-amino-3-alkyl-1,2,4-triazole-5-thione) complexes of first row transition metals: Crystal structures of the cobalt and nickel complexes of 4-amino-3-trifluoromethyl-1,2,4-triazole-5-thione. Inorg. Chim. Acta 2001, 314, 194–200. [Google Scholar] [CrossRef]
- Zangrando, E.; Islam, M.T.; Islam, A.A.A.A.; Sheikh, M.C.; Tarafder, M.T.H.; Miyatake, R.; Zahan, R.; Hossain, M.A. Synthesis, characterization and bio-activity of nickel(II) and copper(II) complexes of a bidentate ns schiff base of S-benzyl dithiocarbazate. Inorg. Chim. Acta 2015, 427, 278–284. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Nassar, M.Y.; Aly, H.M.; Moustafa, M.E. Synthesis, characterization, and biological activity of some novel schiff bases and their Co(II) and Ni(II) complexes: A new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2017, 1143, 462–471. [Google Scholar]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M. Selective flotation of chalcopyrite and molybdenite with plasma pre-treatment. Miner. Eng. 2014, 66, 102–111. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulphide minerals, II: Mechanisms of flotation depression of sulfide minerals by thioglycollic acid and citric acid. Miner. Eng. 2004, 17, 865–878. [Google Scholar] [CrossRef]
- Mitchell, T.K.; Nguyen, A.V.; Evans, G.M. Heterocoagulation of chalcopyrite and pyrite minerals in flotation separation. Adv Colloid Interface Sci 2005, 114–115, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Arquero, A.; Mendiola, M.A. Synthesis, spectral and electrochemical properties of divalent metal complexes containing thiohydrazone and thiosemicarbazone ligands. Polyhedron 1996, 15, 1657–1665. [Google Scholar] [CrossRef]
- Qu, X.; Xiao, J.; Liu, G.; Liu, S.; Zhang, Z. Investigation on the flotation behavior and adsorption mechanism of 3-hexyl-4-amino-1,2,4-triazole-5-thione to chalcopyrite. Miner. Eng. 2016, 89, 10–17. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron X-ray photoelectron spectroscopic study of the chalcopyrite leached by moderate thermophiles and mesophiles. Miner. Eng. 2014, 69, 185–195. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulphuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamasaki, K.; Sawada, S. An X-ray photoelectron spectroscopic study of 2-mercaptobenzothiazole metal complexes. Bull. Chem. Soc. Jpn. 2006, 52, 2908–2912. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by tof-sims, XPS, FTIR, contact angle, zeta potential and micro-flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Clark, R.W.; Squattrito, P.J.; Sen, A.K.; Dubey, S.N. Structural trends in a series of divalent transition metal triazole complexes. Inorg. Chim. Acta 1999, 293, 61–69. [Google Scholar] [CrossRef]
- Yang, Y.; Harmer, S.; Chen, M. Synchrotron-based XPS and nexafs study of surface chemical species during electrochemical oxidation of chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar] [CrossRef]
- Abreu, S.B.; Susana, W. ToF-SIMS-derived hydrophobicity in DTP flotation of chalcopyrite: Contact angle distributions in flotation streams. Int. J. Miner. Process. 2011, 98, 35–41. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hart, B. ToF-SIMS studies of surface chemistry of minerals subjected to flotation separation—A review. Miner. Eng. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Kalegowda, Y.; Chan, Y.L.; Wei, D.H.; Harmer, S.L. X-PEEM, XPS and ToF-SIMS characterisation of xanthate induced chalcopyrite flotation: Effect of pulp potential. Surf. Sci. 2015, 635, 70–77. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Z.; Wang, J.; Liu, Q.; Xiao, J.; Zeng, H.; Zhong, H.; Xu, Z. Study of N-isopropoxypropyl-N′-ethoxycarbonyl thiourea adsorption on chalcopyrite using in situ SECM, ToF-SIMS and XPS. J. Colloid Interface Sci. 2015, 437, 42. [Google Scholar] [CrossRef] [PubMed]











| Samples | Atomic Concentration (%) | |||||
|---|---|---|---|---|---|---|
| C | O | N | S | Cu | Fe | |
| chalcopyrite | 25.45 | 20.73 | 0.00 | 27.48 | 18.98 | 7.36 |
| chalcopyrite + TCH | 60.91 | 11.49 | 7.77 | 12. 93 | 4. 71 | 2.18 |
| Δa | 35.46 | −9.24 | 7.77 | −14.55 | −14.27 | −5.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, C.; Yin, Z.; Ahmed Khoso, S.; Sun, W.; Hu, Y. Performance Analysis of Thiocarbonohydrazide as a Novel Selective Depressant for Chalcopyrite in Molybdenite-Chalcopyrite Separation. Minerals 2018, 8, 142. https://doi.org/10.3390/min8040142
Guan C, Yin Z, Ahmed Khoso S, Sun W, Hu Y. Performance Analysis of Thiocarbonohydrazide as a Novel Selective Depressant for Chalcopyrite in Molybdenite-Chalcopyrite Separation. Minerals. 2018; 8(4):142. https://doi.org/10.3390/min8040142
Chicago/Turabian StyleGuan, Changping, Zhigang Yin, Sultan Ahmed Khoso, Wei Sun, and Yuehua Hu. 2018. "Performance Analysis of Thiocarbonohydrazide as a Novel Selective Depressant for Chalcopyrite in Molybdenite-Chalcopyrite Separation" Minerals 8, no. 4: 142. https://doi.org/10.3390/min8040142
APA StyleGuan, C., Yin, Z., Ahmed Khoso, S., Sun, W., & Hu, Y. (2018). Performance Analysis of Thiocarbonohydrazide as a Novel Selective Depressant for Chalcopyrite in Molybdenite-Chalcopyrite Separation. Minerals, 8(4), 142. https://doi.org/10.3390/min8040142