Chemical and Mineralogical Characterization of Recycled Aggregates from Construction and Demolition Waste from Mexico City
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Methods
2.2.1. Granulometric Characterization
2.2.2. Chemical and Mineralogical Characterization
3. Results and Discussion
3.1. Particle Size Distribution
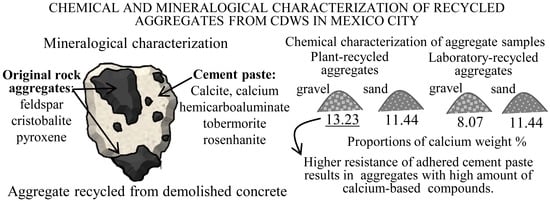
3.2. Mineralogical Composition
3.2.1. Minerals of the Original Aggregates
3.2.2. Minerals of the Adhered Cement Paste
3.3. Chemical Composition and pH
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Norma Ambiental NADF-007-RNAT-2013. Que Establece la Clasificación y Especificaciones de Manejo Para Residuos de la Construcción y Demolición, en el Distrito Federal. Secretaria del Medio Ambiente del Distrito Federal. Available online: www.ordenjuridico.gob.mx (accessed on 19 July 2016).
- Zhao, Z.; Remond, S.; Damidot, D.; Xu, W. Influence of fine recycled concrete aggregates on the properties of mortars. Constr. Build. Mater. 2015, 80, 179–186. [Google Scholar] [CrossRef]
- Blengini, G.A.; Garbarino, E. Resources and waste management in Turin (Italy): The role of recycled aggregates in the sustainable supply mix. J. Clean. Prod. 2010, 18, 1021–1030. [Google Scholar] [CrossRef]
- Rivera-Mera, C.J. Análisis de Impacto Ambiental Por la Inadecuada Disposición de Residuos de la Construcción y Demolición en el Valle de México y Propuestas de Solución. Ph.D. Thesis, Facultad de Ingeniería, UNAM, México City, México, 2007. [Google Scholar]
- Cardoso, R.; Vasco Silva, R.; de Brito, J.; Dhir, R. Use of recycled aggregates from construction and demolition waste in geotechnical applications: A literature review. Waste Manag. 2016, 49, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Norma Mexicana NMX C-170-ONNCCE-1997. Agregados-Reducción de las Muestras de Agregados Obtenidos en el Campo al Tamaño Requerido de las Pruebas. Organismo Nacional de Normalización y Certificación de la Construcción y Edificación, S.C. Available online: www.onncce.org.mx (accessed on 18 February 2017).
- LS-602, (2001), Method of Test for Sieve Analysis of Aggregates. Ministry of Transportation, Ontario, Laboratory Testing Manual. Available online: www.roadauthority.com (accessed on 6 May 2017).
- Norma Mexicana NMX C-077-ONNCCE-1997. Agregados Para Concreto, Análisis Granulométrico, Métodos de Prueba. Organismo Nacional de Normalización y Certificación de la Construcción y Edificación, S.C. Available online: www.onncce.org.mx (accessed on 18 February 2017).
- Norma Mexicana NMX C-111-ONNCCE-2014. Agregados Para Concreto Hidráulico, Análisis Granulométrico, Métodos de Prueba. Organismo Nacional de Normalización y Certificación de la Construcción y Edificación, S.C. Available online: www.onncce.org.mx (accessed on 18 February 2017).
- Gonzalez-Fonteboa, B. Hormigones con áridos reciclados procedentes de demoliciones: Dosificaciones, propiedades mecánicas y comportamiento estructural a cortante. Ph.D. Thesis, Departamento de Tecnología de la Construcción, Universidad Da Coruña, A Coruña, España, 2002. [Google Scholar]
- Kobayashi, S.; Kawano, H. Properties and Usage of Recycled Aggregate Concrete. Demolition and Reuse of Concrete and Masonry: Reuse of Demolition Waste; Chapman and Hall: London, UK, 1988; pp. 547–556. [Google Scholar]
- Vázquez, E.; Barra, M. Reciclaje y Reutilización del Hormigón. In Monografía CIMNE No. 67: Desarrollo Sostenible del Cementy del Hormigón; Gettu, R., Ed.; International Center for Numerical Methods in Engineering: Barcelona, Spain, 2002; pp. 43–65. [Google Scholar]
- Bianchini, G.; Marrocchino, E.; Tassinari, R.; Vaccaro, C. Recycling of construction and demolition waste materials: A chemical-mineralogical appraisal. Waste Manag. 2005, 25, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Guedes, M.; De Brito, J.; Ferro, A.C.; Pereira, M.F. Physical, chemical and mineralogical properties of fine recycled aggregates made from concrete waste. Constr. Build. Mater. 2015, 86, 178–188. [Google Scholar] [CrossRef]
- Bustillo, M. Manual de RCD y Áridos Reciclados; Fueyo Editores: Madrid, Spain, 2010; pp. 641–649. [Google Scholar]
- Omary, S.; Ghorbel, E.; Wardeh, G. Relationships between recycled concrete aggregates characteristics and recycled aggregates concretes properties. Constr. Build. Mater. 2016, 108, 163–174. [Google Scholar] [CrossRef]
- Rodrigues, F.; Carballo, M.T.; Evangelista, L.; De Brito, J. Physical-chemical and mineralogical characterization of fine aggregates from construction and demolition waste recycling plants. J. Clean. Prod. 2013, 52, 438–445. [Google Scholar] [CrossRef]
- Carta Geológica-Minera Ciudad de México E14-2, Esc.1:250,000. Segunda Edición 1997. Servicio Geológico Mexicano. Available online: www.sgm.gob.mx (accessed on 07 August 2017).
- Shi, C.; Li, Y.; Zhang, J.; Li, W.; Chong, L.; Xie, Z. Performance enhancement of recycled aggregate—A review. J. Clean. Prod. 2016, 112, 466–472. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, T.; Liu, S.; Yang, D.; Yu, Q. Investigation of using hybrid recycled powder from demolished concrete solids and clay bricks as a pozzolanic supplement for cement. Constr. Build. Mater. 2014, 73, 754–763. [Google Scholar] [CrossRef]
- Mymrin, V.A.; Alekseev, K.P.; Catai, R.E.; Izzo, R.L.S.; Rose, J.L.; Nagalli, A.; Romano, C.A. Construction material from construction and demolition debris and lime production wastes. Constr. Build. Mater. 2015, 79, 207–213. [Google Scholar] [CrossRef]
- Angulo, S.C.; Ulsen, C.; John, V.M.; Khan, H.; Cincotto, M.A. Chemical-mineralogical characterization of C&D waste recycled aggregates from Sao Paulo, Brazil. Waste Manag. 2009, 29, 721–730. [Google Scholar] [PubMed]
- Catinaud, S.; Beaudoin, J.J.; Marchand, J. Influence of limestone addition on calcium leaching mechanism in cement-based materials. Cem. Concr. Res. 2000, 30, 1961–1968. [Google Scholar] [CrossRef]
- Runcevski, T.; Dinnebier, R.E.; Magdysyuk, O.V.; Pollmann, H. Crystal structures of calcium hemicarboaluminate and carbonated calcium hemicarboaluminate from synchrotron powder diffraction data. Acta Crystallogr. Sect. B 2012, B68, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.P.; Matschei, T. Interactions between portland cement and carbon dioxide. In Proceedings of the 12th International Congress on the Chemistry of Cement-ICCC, Montreal, QC, Canada, 8–13 July 2007. [Google Scholar]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Rahhal, V.F.; Irassar, E.F. Studies on the carboaluminate formation in limestone filler-blended cements. Cem. Concr. Res. 2001, 31, 853–859. [Google Scholar] [CrossRef]
- Manzano, H.; González-Teresa, R.; Dolado, J.S.; Ayuela, A. Espectros de rayos X y propiedades elásticas teóricas de los silicatos cálcicos hidratados cristalinos: comparación con los geles de cemento. Mater. Constr. 2010, 299, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Krajci, L.; Janotka, I. Measurement techniques for rapid assessment of carbonation in concrete. ACI Mater. J. 2000, 97, 168–171. [Google Scholar]
- Lagerblad, B. Carbon Dioxide Uptake during Concrete Life Cycle-State of Art, Report 2:2005; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 2005. [Google Scholar]
- Gaucher, E.C.; Blanc, P. Cement/clay interaction—A review: Experiments, natural analogues, and modeling. Waste Manag. 2006, 26, 776–788. [Google Scholar] [CrossRef] [PubMed]







| Concretos Reciclados S.A. Recycling Plant | Concrete from Landfills | ||
|---|---|---|---|
| Plant-Recycled Gravel | Plant-Recycled Sand | Laboratory-Recycled Gravel | Laboratory-Recycled Sand |
| 109.16 kg | 126.37 kg | 55.24 kg | 125.59 kg |
| Element | Concretos Reciclados S.A. | Concrete from Landfills | ||
|---|---|---|---|---|
| Plant-Recycled Gravel | Plant-Recycled Sand | Laboratory-Recycled Gravel | Laboratory-Recycled Sand | |
| CaO | 23.31 | 19.62 | 14.03 | 12.33 |
| SiO2 | 51.08 | 51.53 | 56.64 | 59.63 |
| Al2O3 | 13.23 | 13.73 | 14.68 | 14.30 |
| Fe0 | 4.52 | 5.20 | 5.18 | 4.66 |
| NaO2 | 3.04 | 4.18 | 4.69 | 4.39 |
| MgO | 1.83 | 1.85 | 1.23 | 1.50 |
| K2O | 1.60 | 1.75 | 1.91 | 2.06 |
| TiO2 | 0.55 | 0.66 | 0.70 | 0.48 |
| S | 0.84 | 1.48 | 0.94 | 0.65 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Oxides | Concretos Reciclados S.A. | Concrete from Landfills | ||
|---|---|---|---|---|
| Plant-Recycled Gravel | Plant-Recycled Sand | Laboratory-Recycled Gravel | Laboratory-Recycled Sand | |
| CaO | 19.11 | 15.76 | 9.78 | 12.33 |
| SiO2 | 49.41 | 50.76 | 55.75 | 56.98 |
| Al2O3 | 8.44 | 9.25 | 10.52 | 10.84 |
| FeO | 3.99 | 4.40 | 4.59 | 4.21 |
| NaO2 | ||||
| MgO | 1.78 | 1.78 | 1.76 | 1.77 |
| K2O | 1.30 | 1.56 | 1.53 | 1.51 |
| TiO2 | 0.49 | 0.54 | 0.48 | 0.49 |
| S | 0.41 | 0.52 | 0.37 | 0.46 |
| Concretos Reciclados S.A. | Concrete from Landfills | |||
|---|---|---|---|---|
| Plant-Recycled Gravel | Plant-Recycled Sand | Laboratory-Recycled Gravel | Laboratory-Recycled Sand | |
| pH | 12.08 | 11.09 | 10.15 | 10.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Pérez, E.; Hernández-Ávila, J.; Rangel-Martínez, Y.; Cerecedo-Sáenz, E.; Arenas-Flores, A.; Reyes-Valderrama, M.I.; Salinas-Rodríguez, E. Chemical and Mineralogical Characterization of Recycled Aggregates from Construction and Demolition Waste from Mexico City. Minerals 2018, 8, 237. https://doi.org/10.3390/min8060237
Moreno-Pérez E, Hernández-Ávila J, Rangel-Martínez Y, Cerecedo-Sáenz E, Arenas-Flores A, Reyes-Valderrama MI, Salinas-Rodríguez E. Chemical and Mineralogical Characterization of Recycled Aggregates from Construction and Demolition Waste from Mexico City. Minerals. 2018; 8(6):237. https://doi.org/10.3390/min8060237
Chicago/Turabian StyleMoreno-Pérez, Emiliano, Juan Hernández-Ávila, Yamile Rangel-Martínez, Eduardo Cerecedo-Sáenz, Alberto Arenas-Flores, Ma. Isabel Reyes-Valderrama, and Eleazar Salinas-Rodríguez. 2018. "Chemical and Mineralogical Characterization of Recycled Aggregates from Construction and Demolition Waste from Mexico City" Minerals 8, no. 6: 237. https://doi.org/10.3390/min8060237
APA StyleMoreno-Pérez, E., Hernández-Ávila, J., Rangel-Martínez, Y., Cerecedo-Sáenz, E., Arenas-Flores, A., Reyes-Valderrama, M. I., & Salinas-Rodríguez, E. (2018). Chemical and Mineralogical Characterization of Recycled Aggregates from Construction and Demolition Waste from Mexico City. Minerals, 8(6), 237. https://doi.org/10.3390/min8060237