Mineralogical and Geochemical Signatures of Metalliferous Sediments in Wocan-1 and Wocan-2 Hydrothermal Sites on the Carlsberg Ridge, Indian Ocean
Abstract
:1. Introduction
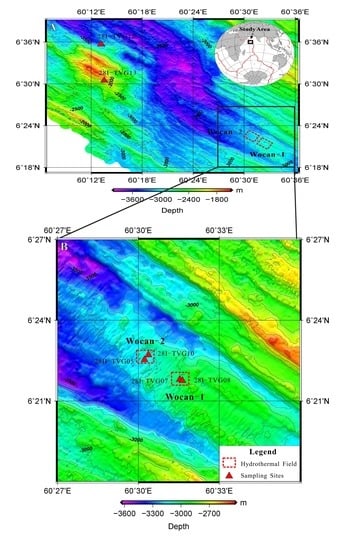
2. Materials and Methods
3. Results
3.1. Mineralogy and Morphology of Sulfide and Non-Sulfide Grains
3.2. Bulk Sediment Geochemistry
3.3. Mineral Chemistry of Sulfide Separates
3.4. Sulfur Isotopes
4. Discussion
4.1. The Effect of Primary and Post-Depositional Changes, Evidence from Grain Texture, Bulk Chemical Compositions, and Mineral Chemistry
4.2. The Contributions of Hydrothermal Fluid, Evidence from S Isotope Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

Appendix C

Appendix D

References
- Edmond, J.M.; Measures, C.; McDuff, R.E.; Chan, L.H.; Collier, R.; Grant, B.; Gordon, L.I.; Corliss, J.B. Ridge crest hydrothermal activity and the balances of the major and minor elements in the ocean: The Galapagos data. Earth Planet. Sci. Lett. 1979, 46, 1–18. [Google Scholar] [CrossRef]
- Kadko, D.; Baross, J.; Alt, J.C. The Magnitude and Global Implications of Hydrothermal Flux. In Geophysical Monograph Series; American Geophysical Union: Washington, DC, USA, 1995; Volume 91, pp. 446–466. 091p. [Google Scholar]
- Dias, Á.S.; Barriga, F.J.A.S. Mineralogy and geochemistry of hydrothermal sediments from the serpentinite-hosted Saldanha hydrothermal field (36°34′ N; 33°26′ W) at MAR. Mar. Geol. 2006, 225, 157–175. [Google Scholar] [CrossRef]
- Baker, E.T.; German, C.R.; Elderfield, H. Hydrothermal plumes over Spreading-center axes: Global distribution and geological inferences. In Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geological Interactions; American Geophysical Union: Washington, DC, USA, 1995; pp. 47–71. [Google Scholar] [CrossRef]
- Baker, E.T.; German, C.R. On the global distribution of hydrothermal vent Fields. In Mid-Ocean Ridges: Hydrothermal Interactions between the Lithosphere and Oceans; German, C.R., Lin, J., Parson, L., Eds.; American Geophysical Union: Washington, DC, USA, 2004; pp. 245–266. [Google Scholar]
- De Martins, B.J.; Sohn, R.A.; Canales, P.J.; Humphris, S.E. Kinematics and geometry of active detachment faulting beneath the Trans-Atlantic Geotraverse (TAG) hydrothermal field on the Mid-Atlantic Ridge. Geology 2007, 35, 711–714. [Google Scholar] [CrossRef]
- Mills, R.A. Hydrothermal deposits and metalliferous sediments from TAG, 26° N Mid-Atlantic Ridge. Geol. Soc. Lond. Spéc. Publ. 1995, 87, 121–132. [Google Scholar] [CrossRef]
- Dias, Á.S.; Mills, R.A.; Taylor, R.N.; Ferreira, P.; Barriga, F.J.A.S. Geochemistry of a sediment push-core from the Lucky Strike hydrothermal field, Mid-Atlantic Ridge. Chem. Geol. 2008, 247, 339–351. [Google Scholar] [CrossRef]
- Ikehata, K.; Suzuki, R.; Shimada, K.; Ishibashi, J.; Urabe, T. Mineralogical and Geochemical Characteristics of Hydrothermal Minerals Collected from Hydrothermal Vent Fields in the Southern Mariana Spreading Center. In Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept; Ishibashi, J.-I., Okino, K., Sunamura, M., Eds.; Springer: Japan, Tokyo, 2015; pp. 275–287. [Google Scholar] [CrossRef]
- Humphris, S.E.; Klein, F. Progress in Deciphering the Controls on the Geochemistry of Fluids in Seafloor Hydrothermal Systems. Annu. Rev. Mar. Sci. 2018, 10, 315–343. [Google Scholar] [CrossRef] [PubMed]
- Gurvich, E.G. Metalliferous Sediments of the World Ocean: Fundamental Theory of Deep-Sea Hydrothermal Sedimentation; Springer: Berlin, Germany, 2006. [Google Scholar]
- Dias, A.S. Geochemistry of Deep-Sea Hydrothermal Sediments from the Saldanha and Lucky Strike Hydrothermal Fields (Mid-Atlantic Ridge), Universidade de Lisboa. 2009. Available online: https://core.ac.uk/display/12421951 (accessed on 12 August 2018).
- Mills, R.A.; Elderfield, H. Rare Earth Element geochemistry of hydrothermal deposits from the active TAG Mound 26° N Mid-Atlantic Ridge. Geochem. Cosmochim. Acta 1995, 59, 3511–3524. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Petersen, S.; Frische, M.; Qiu, Z.; Li, H.; Li, H.; Wu, Z.; Cui, R. Mineralogy and trace element geochemistry of sulfide minerals from the Wocan Hydrothermal Field on the slow-spreading Carlsberg Ridge, Indian Ocean. Ore Geol. Rev. 2017, 84, 1–19. [Google Scholar] [CrossRef]
- Qiu, Z.; Han, X.; Wang, Y. Manned deep submarine observation of modern seafloor hydrothermal activities in Northwest India Ocean, Abstract of proceedings at the 5th international sedimentological conference, Nanjing, China, 2017.
- Jin, C.; Li, C.; Algeo, T.J.; Planavsky, N.J.; Cui, H.; Yang, X.; Zhao, Y.; Zhang, X.; Xie, S. A highly redox-heterogeneous ocean in South China during the early Cambrian (∼529–514 Ma): Implications for biota-environment co-evolution. Earth Planet. Sci. Lett. 2016, 441, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Keith, M.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R.; Petersen, S.; Bach, W. Effects of temperature, sulfur and oxygen fugacity on the composition of sphalerite from submarine hydrothermal vents. Geology 2014, 48, 699–702. [Google Scholar] [CrossRef]
- German, C.R.; Higgs, N.C.; Thomson, J.; Mills, R.; Elderfield, H.; Blusztajn, J.; Fleer, A.P.; Bacon, M.P. A geochemical study of metalliferous sediment from the TAG hydrothermal mound, 26°8′ N, Mid-Atlantic Ridge. J. Geophys. Res. 1993, 98, 9683–9692. [Google Scholar] [CrossRef]
- Mills, R.A.; Thomson, J.; Elderfield, H.; Hinton, R.W.; Hyslop, E. Uranium enrichment in metalliferous sediments from the Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 1994, 124, 35–47. [Google Scholar] [CrossRef]
- German, C.R.; Hergt, J.; Palmer, M.R.; Edmond, J.M. Geochemistry of a hydrothermal sediment core from the OBS vent-field, 21° N East Pacific Rise. Chem. Geol. 1999, 155, 65–75. [Google Scholar] [CrossRef]
- Hannington, M.D.; Tivey, M.K.; Larocque, A.C.L.; Petersen, S.; Rona, P.A. The Occurrence of gold in sulfide deposits of the TAG hydrothermal field, Mid-Atlantic Ridge. Can. Miner. 1995, 33, 1285–1310. [Google Scholar]
- Hannington, M.D.; Poulsen, K.H.; Thompson, J.F.H.; Sillitoe, R.H. Volcanogenic gold in the massive sulfide environment. In Volcanic-Associated Massive Sulfide Deposits: Processes and Examples in Modern and Ancient Settings; Reviews in Economic Geology; Barrie, C.T., Hannington, M.D., Eds.; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 8, pp. 325–356. [Google Scholar]
- Keith, M.; Smith, D.J.; Jenkin, G.R.T.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- McDermott, J.M.; Ono, S.; Tivey, M.K.; Seewald, J.S.; Shanks, W.C.; Solow, A.R. Identification of sulfur sources and isotopic equilibria in submarine hot-springs using multiple sulfur isotopes. Geochim. Cosmochim. Acta 2015, 160, 169–187. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, J.; Jiang, F.; Zhai, S.; Qin, Y.; Hou, Z. Sulfur isotopic composition of seafloor hydrothermal sediment from the Jade hydrothermal field in the central Okinawa Trough and its geological significance. Acta Oceanol. Sin. 2002, 21, 395–405. [Google Scholar]
- Keith, M.; Haase, K.M.; Klemd, R.; Krumm, S.; Strauss, H. Systematic variations of trace element and sulfur isotope compositions in pyrite with stratigraphic depth in the Skouriotissa volcanic-hosted massive sulfide deposit, Troodos ophiolite, Cyprus. Chem. Geol. 2016, 423, 7–18. [Google Scholar] [CrossRef]
- Zeng, Z.; Ma, Y.; Chen, S.; Selby, D.; Wang, X.; Yin, X. Sulfur and lead isotopic compositions of massive sulfides from deep-sea hydrothermal systems: Implications for ore genesis and fluid circulation. Ore Geol. Rev. 2017, 87, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Herzig, P.M.; Petersen, S.; Hannington, M.D. Geochemistry and sulfur isotopic composition of the TAG hydrothermal mound, Mid-Atlantic Ridge, 26° N. Proc. Ocean Drill. Program Sci. Results 1998, 158, 47–70. [Google Scholar]
- Rees, C.E.; Jenkins, W.J.; Monster, J. The sulphur isotopic composition of ocean water sulphate. Geochim. Cosmochim. Acta 1978, 42, 377–381. [Google Scholar] [CrossRef]
- Gemmell, J.B.; Sharpe, R. Detailed sulfur-isotope investigation of the TAG hydrothermal mound and stock work zone, 26° N, Mid-Atlantic Ridge. Proceedings of the Ocean Drilling Program. Sci. Results 1998, 158, 71–84. [Google Scholar]
- Janecky, D.R.; Shanks, W.C. Computational modelling of chemical and sulfur isotopic reaction processes in seafloor hydrothermal systems: Chimneys, massive sulfides and subjacent alteration zones. Can. Miner. 1988, 26, 805–825. [Google Scholar]
- Lein, A.Y.; Grinenko, V.A.; Ulyanova, N.V.; Lisitsin, A.P. Evolution of Oceanic hydro-thermal system and sulphur isotope composition of sulphides. Geochimya 1991, 3, 307–319. [Google Scholar]
- Butler, B.; Fallick, A.E.; Nesbitt, R.W. Mineralogy, sulphur isotope Geochemistry and the development of sulphide structures at the Broken Spur hydrothermal vent site, 29°10′ N, Mid-Atlantic Ridge. J. Geol. Soc. 1998, 155, 773–785. [Google Scholar] [CrossRef]
- Lehrmann, B.; Stobbs, I.J.; Lusty, P.A.; Murton, B.J. Insights into Extinct Seafloor Massive Sulfide Mounds at the TAG, Mid-Atlantic Ridge. Minerals 2018, 8, 302. [Google Scholar] [CrossRef]
- Buseck, P.R. Structural relationship between pyrite and marcasite. Am. Mineral. 1996, 81, 119–125. [Google Scholar]
- Criddle, A.J.; Stanley, C.J. (Eds.) Quantitative Data File for Ore Minerals, 3rd ed.; Chapman & Hall: London, UK, 1993; Volume 84, Available online: http://handbookofmineralogy.org/pdfs (accessed on 22 December 2018).
- Popoola, S.; Han, X.; Wang, Y.; Qiu, Z.; Ye, Y. Geochemical investigations on Fe–Si–Mn oxyhydroxides deposits in Wocan Field on the slow spreading Carlsberg Ridge, Indian Ocean. Minerals 2019, 9. [Google Scholar] [CrossRef]




| Site | Station | Longitude (E) | Latitude (N) | Depth (m) | Type of Samples |
|---|---|---|---|---|---|
| Wocan-1 | 28I-TVG07 | 60°31.534′ | 6°21.796′ | 2989 | Metalliferous sediment |
| 28I-TVG08 | 60°31.635′ | 6°21.756′ | 2973 | Metalliferous sediment | |
| Wocan-2 | 28I-TVG10 | 60°30.372′ | 6°21.866′ | 3104 | Metalliferous sediment |
| 28II-TVG05 | 60°30.226′ | 6°22.534′ | 3105 | Metalliferous sediment | |
| Ridge flank | 28I-TVG13 | 60°13.190′ | 6°35.675′ | 3254 | Pelagic sediment |
| 28I-TVG12 | 60°13.550′ | 6°30.462′ | 2009 | Pelagic sediment |
| Station | Pyrite | Chalcopyrite | Sphalerite | Secondary Cu-sulfides | Barite | Gypsum/Anhydrite | Fe-oxyhydroxides | Amorphous Silica | Volcanic Glass | Biogenic Calcite |
|---|---|---|---|---|---|---|---|---|---|---|
| Wocan-1 | ||||||||||
| 28I-07 | XXX | XX | X | - | XXX | XX | X | XX | X | |
| 28I-08 | XXX | XXX | X | X | X | XX | XXX | X | X | X |
| Wocan-2 | ||||||||||
| 28I-10 | XX | XXX | X | XX | X | X | XXX | XX | X | X |
| 28II-05 | XX | X | - | - | - | XX | X | X | XX | |
| Ridge flanks | ||||||||||
| 28I-12 | - | - | - | - | - | - | - | - | - | XXX |
| 28I-13 | - | - | - | - | - | - | X | - | - | XXX |
| Element | Wocan1 | Wocan2 | Ridge Flanks | |||
|---|---|---|---|---|---|---|
| TVG-07 | TVG-08 | TVG-05 | TVG-10 | TVG-12 | TVG-13 | |
| Fe (wt.%) | 30.9 | 41.2 | 26 | 31.4 | 0.63 | 1.46 |
| Mg (wt.%) | 1.31 | 0.49 | 0.5 | 0.14 | 0.44 | 0.79 |
| Ca (wt.%) | 1.48 | 0.3 | 9.81 | 0.2 | 31.7 | 29.5 |
| Mn (wt.%) | 0.02 | 0.03 | 0.09 | 0.06 | 0.04 | 0.03 |
| Al (wt.%) | 1.38 | 0.09 | 0.82 | 0.47 | 0.63 | 1.52 |
| Ti (wt.%) | 0.04 | 0.02 | 0.82 | 0.47 | 0.63 | 1.52 |
| S (wt.%) | 7.87 | 10 | 5.24 | 10 | 0.63 | 1.52 |
| Cu (ppm) | 31,100 | 33,100 | 8510 | 51,900 | 60 | 171 |
| Zn (ppm) | 4710 | 8330 | 2890 | 6750 | 47 | 24 |
| Pb (ppm) | 431 | 430 | 158 | 404 | 5.3 | 5.4 |
| Ag (ppm) | 9.66 | 15.85 | 3.4 | 16.25 | 0.25 | 0.09 |
| Co (ppm) | 20.5 | 31.1 | 235 | 16.7 | 4.6 | 7.7 |
| Cr (ppm) | 32 | 22 | 22 | 17 | 10 | 25 |
| Ba (ppm) | 40 | 80 | 190 | 30 | 600 | 500 |
| U (ppm) | 4.19 | 7.4 | 4.7 | 19.6 | 0.4 | 0.5 |
| Th (ppm) | 0.5 | 0.2 | 1.1 | 0.2 | 1 | 1.3 |
| V (ppm) | 225 | 150 | 266 | 225 | 16 | 28 |
| Fe/Mn | 1545 | 1373 | 288 | 523 | 17 | 47 |
| U/Fe | 1.36 × 10−5 | 1.80 × 10−5 | 1.81 × 10−5 | 6.24 × 10−5 | 6.35 × 10−5 | 3.42 × 10−5 |
| Cu +Fe + Zn (wt.%) | 34.48 | 45.34 | 27.14 | 37.27 | 0.64 | 1.48 |
| (wt.%) | Station | S | Fe | Se | Cu | Co | Zn | Sb | Ag | Pb | Sn | Tl | Total | Cu/Fe Atom | ||
| Chalcopyrite | Min | 34.26 | 30.41 | 0.01 | 32.69 | 0.02 | bdl | 0.01 | 0.01 | 0.05 | bdl | bdl | 97.53 | 0.99 | ||
| Max | 36.49 | 30.86 | 0.03 | 35.12 | 0.04 | 0.02 | 0.03 | 0.01 | 0.12 | bdl | bdl | 101.73 | 1.01 | |||
| n = 11 | Av | 35.43 | 30.33 | 0.02 | 34.46 | 0.03 | 0.02 | 0.01 | 0.08 | 100.38 | 1.00 | |||||
| Wocan-1 | STDEV | ±0.59 | ±0.46 | ±0.01 | ±0.91 | ±0.00 | ±0.00 | ±0.02 | ±1.2 | ±0.01 | ||||||
| Chalcopyrite | Min | 34.79 | 30.13 | bdl | 34.47 | bdl | 0.01 | bdl | bdl | 0.03 | 0.02 | 0.02 | 99.47 | 0.98 | ||
| Max | 35.56 | 30.98 | bdl | 35.00 | bdl | 0.06 | bdl | 0.07 | 0.07 | 0.03 | 0.04 | 101.81 | 1.01 | |||
| n = 12 | Av | 35.13 | 30.57 | 34.75 | 0.03 | 0.05 | 0.02 | 0.03 | 100.58 | 0.99 | ||||||
| Wocan-2 | STDEV | ±0.24 | ±0.28 | ±0.18 | ±0.01 | ±0.01 | ±0.01 | ±0.01 | ±0.38 | ±0.01 | ||||||
| (wt.%) | Station | S | Fe | As | Se | Cu | Co | Zn | Sb | Ag | Pb | Bi | Au | Ni | S/Fe Atom | Total |
| Pyrite | Min | 51.70 | 45.19 | 0.02 | 0.02 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.30 | bdl | 0.01 | bdl | 1.94 | 97.14 |
| Max | 53.17 | 46.84 | 0.31 | 0.05 | 0.36 | 0.06 | 0.02 | 0.01 | 0.12 | 0.06 | 0.02 | 0.04 | 2.05 | 101.23 | ||
| n = 26 | Av | 53.15 | 46.36 | 0.11 | 0.03 | 0.08 | 0.05 | 0.01 | 0.01 | 0.15 | 0.01 | 2.00 | 99.97 | |||
| Wocan-1 | STDEV | ±0.65 | ±0.33 | ±0.12 | ±0.01 | ±0.11 | ±0.00 | ±0.00 | ±0.00 | ±0.04 | ±0.03 | ±0.77 | ||||
| Pyrite | Min | 51.87 | 46.12 | 0.10 | bdl | 0.01 | bdl | bdl | bdl | bdl | 0.04 | bdl | bdl | 1.96 | 98.14 | |
| Max | 53.97 | 46.88 | 0.13 | bdl | 0.17 | bdl | 0.02 | bdl | bdl | 0.08 | bdl | 0.01 | 2.01 | 101.26 | ||
| n = 11 | Av | 53.14 | 46.54 | 0.12 | 0.08 | 0.06 | 1.99 | 99.94 | ||||||||
| Wocan-2 | STDEV | ±0.59 | ±0.25 | ±0.02 | ±0.05 | ±0.02 | ±0.01 | ±0.01 | 0.01 | ±0.78 | ||||||
| (wt.%) | Station | S | Fe | Se | Cu | Mo | Zn | Cd | Pb | Total | Fe/Zn | T (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphalerite | Min | 33.92 | 4.04 | 0.07 | 0.01 | 61.08 | 0.46 | 0.04 | 99.62 | 0.06 | 278.03 | |
| Max | 34.20 | 4.06 | 0.01 | 0.09 | 0.04 | 61.29 | 0.50 | 0.05 | 100.24 | 0.07 | 278.15 | |
| n = 3 | Av | 34.09 | 4.05 | 0.10 | 0.02 | 61.22 | 0.48 | 0.05 | 100.01 | 0.07 | 278.08 | |
| Wocan-1 | STDEV | ±0.14 | ±0.33 | ±0.02 | ±0.01 | ±0.11 | ±0.02 | ±0.00 | ±0.27 | ±0.00 | ±0.06 | |
| Sphalerite | Min | 33.40 | 0.19 | bdl | 0.01 | 0.02 | 64.11 | 0.20 | 0.01 | 97.94 | 0.00 | 229.40 |
| Max | 32.75 | 1.36 | 0.01 | 0.06 | 0.04 | 66.45 | 0.31 | 0.04 | 101.02 | 0.02 | 243.47 | |
| n = 5 | Av | 33.28 | 0.47 | 0.03 | 0.03 | 65.14 | 0.27 | 0.03 | 99.25 | 0.01 | 232.73 | |
| Wocan-2 | STDEV | ±0.56 | ±0.50 | ±0.02 | ±0.00 | ±0.90 | ±0.05 | ±0.01 | ±0.78 | ±0.00 | ±6.02 |
| Station | δ34Spy-VCDT (‰) | Seawater Derived (%) | Magmatic Origin (%) |
|---|---|---|---|
| TVG08 | 3.0 | 14.3 | 85.7 |
| TVG07 | 3.6 | 17.1 | 82.9 |
| TVG07 | 3.6 | 17.1 | 82.9 |
| TVG07 | 3.6 | 17.1 | 82.9 |
| TVG10 | 4.1 | 19.5 | 80.5 |
| TVG10 | 4.3 | 20.5 | 79.5 |
| TVG10 | 4.3 | 20.5 | 79.5 |
| TVG05 | 6.4 | 30.5 | 69.5 |
| TVG05 | 7.9 | 37.6 | 62.4 |
| TVG05 | 8.7 | 41.4 | 58.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popoola, S.O.; Han, X.; Wang, Y.; Qiu, Z.; Ye, Y.; Cai, Y. Mineralogical and Geochemical Signatures of Metalliferous Sediments in Wocan-1 and Wocan-2 Hydrothermal Sites on the Carlsberg Ridge, Indian Ocean. Minerals 2019, 9, 26. https://doi.org/10.3390/min9010026
Popoola SO, Han X, Wang Y, Qiu Z, Ye Y, Cai Y. Mineralogical and Geochemical Signatures of Metalliferous Sediments in Wocan-1 and Wocan-2 Hydrothermal Sites on the Carlsberg Ridge, Indian Ocean. Minerals. 2019; 9(1):26. https://doi.org/10.3390/min9010026
Chicago/Turabian StylePopoola, Samuel Olatunde, Xiqiu Han, Yejian Wang, Zhongyan Qiu, Ying Ye, and Yiyang Cai. 2019. "Mineralogical and Geochemical Signatures of Metalliferous Sediments in Wocan-1 and Wocan-2 Hydrothermal Sites on the Carlsberg Ridge, Indian Ocean" Minerals 9, no. 1: 26. https://doi.org/10.3390/min9010026
APA StylePopoola, S. O., Han, X., Wang, Y., Qiu, Z., Ye, Y., & Cai, Y. (2019). Mineralogical and Geochemical Signatures of Metalliferous Sediments in Wocan-1 and Wocan-2 Hydrothermal Sites on the Carlsberg Ridge, Indian Ocean. Minerals, 9(1), 26. https://doi.org/10.3390/min9010026